Amazon censored this book until recently, so I thought I’d give it some extra airtime.
The more people that understand Cartel Medicine’s cancer racket the better. And the more people that understand there are many, many other ways of surviving cancer without poisoning, burning or cutting, the better.
With thanks to Dr. Paul Marik.
Let’s first look at an analogy born out of the ideas in the book.
Podcast Conversation
Analogy: The City Under Siege
Imagine a thriving city (the healthy body) protected by an advanced defense system (immune system) and powered by efficient power plants (mitochondria). Cancer development is like a gradual subversion of this city's infrastructure, where:
The city's power plants (mitochondria) begin malfunctioning, forcing buildings (cells) to use emergency generators (glycolysis) that are less efficient but allow survival. These compromised buildings start ignoring the city's building codes (normal cell regulation) and begin constructing unauthorized additions (uncontrolled growth).The city's main districts (tissues) become infiltrated by these rogue buildings, which:
Create their own infrastructure (angiogenesis)
Establish underground networks (metastasis pathways)
Corrupt the local police force (immune system)
Hack into the city's communication system (cell signaling)
The city's defense system faces several challenges:
Rogue buildings disguise themselves as legitimate structures (immune evasion)
Some buildings become master architects (cancer stem cells), able to rebuild entire unauthorized districts
The rebels create protective neighborhoods (tumor microenvironment) where city laws don't apply
Traditional cancer treatments are like different military strategies:
Chemotherapy: Carpet bombing that damages both rebel and loyal buildings
Radiation: Precision strikes on specific districts
Immunotherapy: Retraining and reinforcing the city's police force
Targeted therapy: Cutting off specific supply lines or communication networks
Metabolic therapy: Disrupting the rebels' power supply while protecting the city's infrastructure
The city's survival depends on:
Early detection of rebel activities
Understanding the rebels' unique vulnerabilities
Protecting critical infrastructure
Maintaining supply lines for loyal citizens
Coordinating multiple response strategies
Preventing the spread to other cities (metastasis)
This analogy illustrates why cancer is so challenging to treat - it's not an external invader but a complex internal rebellion that uses the body's own systems to survive and spread, requiring sophisticated, multi-targeted approaches for effective treatment.
20-point summary
Cancer development involves complex interactions between metabolic dysfunction, genetic mutations, and environmental factors, with increasing evidence supporting the primary role of metabolic alterations as initiating events rather than just consequences.
The Warburg effect demonstrates cancer cells' unique metabolism, preferring glycolysis even in the presence of oxygen, making metabolic targeting a promising therapeutic approach.
Tumor heterogeneity presents a major challenge in treatment, as different cell populations within the same tumor can exhibit varying responses to therapy, necessitating multi-targeted approaches.
Cancer stem cells serve as key drivers of tumor initiation, progression, and treatment resistance, making them crucial therapeutic targets for preventing recurrence.
The tumor microenvironment significantly influences treatment outcomes through complex interactions between cancer cells, immune cells, blood vessels, and surrounding tissues.
Immunotherapy has emerged as a revolutionary treatment approach, though its success depends heavily on individual patient factors and specific tumor characteristics.
Early detection remains crucial for optimal outcomes, with significant survival differences between early and late-stage diagnoses across most cancer types.
Treatment resistance develops through multiple mechanisms, including genetic adaptation, metabolic rewiring, and microenvironmental changes, requiring adaptive treatment strategies.
Personalized medicine approaches, based on molecular profiling and biomarker testing, are increasingly important for optimizing treatment selection and outcomes.
The economic aspects of cancer treatment, including drug development costs and healthcare access, significantly impact treatment availability and patient outcomes.
Clinical trial design and regulation play crucial roles in treatment development, though current systems may inadvertently discourage research into certain promising approaches.
Environmental and lifestyle factors, including diet, stress, and physical activity, significantly influence both cancer risk and treatment outcomes.
Epigenetic modifications provide a crucial link between environmental exposures and cancer development, offering potential therapeutic targets.
The press-pulse therapeutic approach represents an innovative strategy targeting cancer's metabolic vulnerabilities while minimizing damage to healthy cells.
Quality of life considerations increasingly influence treatment decisions, balancing survival benefits against side effects and functional impacts.
International research standards and regulatory processes affect global treatment development and accessibility, creating both opportunities and challenges.
The role of inflammation in cancer development and progression has led to new therapeutic strategies targeting inflammatory pathways.
Advanced imaging technologies and biomarker testing have revolutionized diagnosis, monitoring, and treatment selection.
The integration of multiple treatment modalities, including conventional, targeted, and metabolic approaches, offers the most promising path forward for many cancers.
Understanding the complex relationship between stress, immune function, and cancer progression has led to increased emphasis on comprehensive care approaches including psychological support.
FOREWORD by Dr. Justus Hope
As a physician and board-certified specialist, I have spent over 30 years caring for patients, mainly those suffering from intractable pain. In January 2020, when my friend contracted glioblastoma, I began researching ways to help him. What I found annoyed me: My friend could do far better if his doctors would add repurposed drug cocktails to his chemotherapy, radiation, and surgery.
A Harvard professor first stumbled upon repurposed drugs for cancer in the 1990s when he used them to cure his own glioblastoma. That man is still alive today.
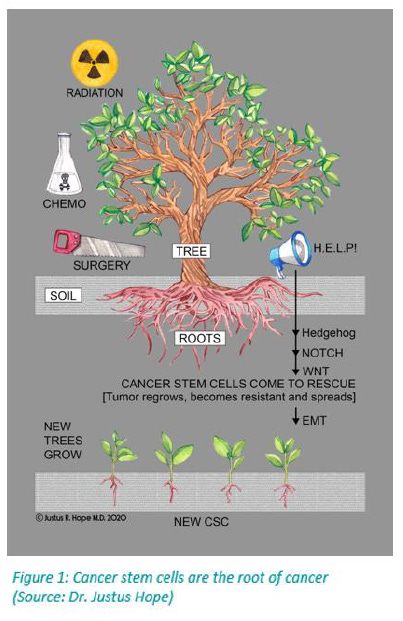
The most significant problem I see repeatedly is that cancer recurs with resistant metastases. At that point, even with repurposed drugs, it is often a losing battle. This tragedy occurs because the standard treatments of surgery, radiation, and chemotherapy stimulate the growth of cancer stem cells (see Figure 1). Proactively adding repurposed drugs as early as possible can help prevent cancer stem cells from regrowing the tumor into a more resistant and sometimes indestructible form. If we could get all patients and their oncologists to read this document and add a repurposed drug cocktail, along with lifestyle changes, at the onset of a cancer diagnosis (and do this in concert with their treatment plan — whether it be surgery, chemotherapy, and radiation treatment) we would likely see a lot more of these patients not only survive but live better, longer lives.
Justus Hope is a pen name. The author practices medicine under his given name. He has written several books, including Surviving Cancer, COVID-19, and Disease: The Repurposed Drug Revolution.
FOREWORD for 2nd EDITION OF CANCER CARE
by Dr. Justus Hope
Dr. Paul Marik, the most published Critical Care Specialist in the United States, has tackled cancer. Dr. Marik reviewed thousands of studies, and in the process, uncovered fundamental truths and busted persistent myths in publishing Cancer Care 1st Edition. This 2nd edition expands these and adds propranolol, a relatively unknown anticancer agent. Moreover, ivermectin is moved up to Tier One based on emerging evidence.
There are numerous practical suggestions, and all are based on PubMed studies so no one can dispute the scientific and evidence basis. Marik gives us some guidance on which three repurposed drugs to take before cancer surgery or biopsy to decrease the risk of spreading the cancer through surgical manipulation. Cutting a tumor has long been known to pose a risk of spreading cancer, yet patients have not been warned of this. Marik notes that studies have shown that these risks can be reduced with the pre-operative use of celecoxib, propranolol, or cimetidine. Better yet, combining all three may have a synergistic effect.
However, my review of the 2nd edition of Dr. Marik’s Cancer provided much more, and I am honored to share it here. First, let me start with the root cause of cancer. Dr. Marik notes the Somatic Mutation Theory, the one we are all taught in medical school, is not supported by the growing data. Cancer, as we have been told for decades, is not caused by a series of mutations that result eventually in out-of-control cell division.
Instead, the data provides much more support for the Mitochondrial Dysfunction Model as espoused by Dr. Thomas Seyfried. When cancer cells’ mitochondria are transplanted into normal cells, the normal cells become cancerous. However, when cancer cells’ nuclei are transplanted into normal cells, the cancer does not transfer. The causative agent is carried in the mitochondria, not in the nucleus. The central issue in cancer is defective mitochondria, not DNA mutations.
Why has this myth that cancer arises from DNA mutations persisted?
Because the Somatic Mutation Theory is the narrative, and the narrative produces expensive and profitable treatments. The results remain lacking. The narrative probably won’t change any time soon, however, Dr. Marik notes that addressing cancer treatment according to the Mitochondrial Dysfunction Model produces different therapies and much better outcomes.
These treatments, which can be done in addition to the standard of care for most patients, can result in longer survival times - in some cases complete remission. Perhaps more importantly, Marik’s recommendations may result in avoiding cancer altogether, a prospect that could demolish the cancer industry’s profits.
But I digress.
Let us get to some of the other myths that Marik busts.
“Sunlight is bad for you as it increases your risk of cancer and sunscreen is healthy for you as it decreases your cancer risk.”
Wrong. It is quite the opposite. In the 2nd Edition, Dr. Marik convincingly shows, via multiple studies, how sunlight reduces melanoma risk and improves survival and how sunscreen does the opposite.
When my friend and colleague developed Glioblastoma, a serious brain cancer with an average survival of 12.7 months, I dove into the medical literature and found repurposed drugs. After publishing a book in 2020 – Surviving Cancer COVID-19 and Disease: The Repurposed Drug Revolution – on my findings including the vast evidence base on Repurposed Drugs for Cancer, my friend added four of these - Atorvastatin, Mebendazole, Metformin, and Doxycycline - to his treatment plan through the US Care Oncology Clinic, and he survived some 46 months - almost 4 times longer than expected.
For this his family was grateful. However, we had all hoped for more. And when he ultimately passed, it was the radiation damage that played the largest role, not the cancer. Dr. Thomas Seyfried has noticed the same issue in his interviews on Glioblastoma. Seyfried explains the brain should never be irradiated.
I only wish we had this information in 2020. We needed Dr. Seyfried’s and Dr. Marik’s cancer knowledge then. My friend could have done better and lived longer.
Which brings me to propranolol. Dr. Marik ranks this second in evidence support only to Vitamin D3. Why? The book covers all the technical and detailed anti-cancer pathways of propranolol. However, the gist of it is this:
Propranolol is a beta blocker that shields the effect of catecholamines on the body. Catecholamines like norepinephrine and epinephrine are released when we encounter stress, and stress increases the likelihood of developing cancer. Therefore, by blocking the catecholamines, propranolol reduces the risk of developing cancer.
However, the key property of this beta blocker is that it reduces metastatic spread.
This brings me to one of Dr. Marik’s favorite parts of his upcoming book. Three repurposed drugs can offer preventative effects against metastatic spread in those undergoing cancer surgery. These include celecoxib, cimetidine, and propranolol. Dr. Marik writes the combination may be synergistic.
Dr. Paul Marik has taken the science of Repurposed Drugs in Cancer to a world-class level. Cancer Care 1st Edition is worthy of a PhD thesis with more than 1200 PubMed citations. Marik has earned another PhD with more than 1300 references in this new edition. The most published Critical Care Specialist in the United States has become the most credible scientific voice for repurposed drugs in preventing and treating Cancer.
And on a personal note, talking with Dr. Marik has been a pleasant adventure. Speaking with him is like talking to your best friend albeit with a South African accent and an AI-like knowledge of the subject. He has the most remarkable combination of intellect, compassion, and humility of anyone I know.
The 1st and 2nd editions of Dr. Marik’s Cancer Care will change how the world approaches cancer.
Justus R. Hope, MD
Redding, California
May 2024
Cancer Care
The role of repurposed drugs and metabolic interventions in treating cancer
Paul E. Marik, MD, FCCM, FCCP
50 Questions & Answers
Question 1: How does the mitochondrial dysfunction model differ from the somatic mutation theory in explaining cancer development?
Answer: The mitochondrial dysfunction model posits that cancer originates from damaged cellular respiration in mitochondria, leading to compensatory fermentation and metabolic changes that drive cancer development. This model, supported by researchers like Thomas Seyfried, suggests that genetic mutations are a downstream effect rather than the primary cause of cancer.
In contrast, the somatic mutation theory views cancer as primarily caused by DNA mutations that accumulate over time, leading to uncontrolled cell growth. While both models acknowledge the presence of genetic alterations, they fundamentally differ in their understanding of causation - the mitochondrial model sees metabolic dysfunction as the initiator, while the mutation theory sees genetic changes as the trigger.
Question 2: What is the Warburg effect and how does it relate to cancer cell metabolism?
Answer: The Warburg effect describes the unique metabolic pattern observed in cancer cells where they preferentially use glycolysis for energy production even in the presence of oxygen, a phenomenon first documented by Otto Warburg. This process, also known as aerobic glycolysis, is less efficient than normal oxidative phosphorylation but provides cancer cells with advantages for rapid growth and survival.
This metabolic shift allows cancer cells to produce energy and building blocks for new cells while maintaining a favorable microenvironment for tumor growth. The increased glucose consumption and lactate production associated with the Warburg effect also create an acidic environment that can promote tumor invasion and suppress immune system response.
Question 3: How do press-pulse protocols work in cancer treatment, and what distinguishes them from conventional approaches?
Answer: Press-pulse protocols combine sustained metabolic pressure ("press") with acute stressors ("pulse") to target cancer cells' unique metabolic vulnerabilities. The press phase typically involves dietary modifications or metabolic therapies that create a sustained stress on cancer cells, while the pulse phase introduces periodic acute stressors such as high-dose targeted treatments or hyperbaric oxygen therapy.
This approach differs from conventional treatments by systematically exploiting the metabolic weaknesses of cancer cells while minimizing damage to healthy cells. Unlike traditional chemotherapy or radiation that primarily target rapidly dividing cells, press-pulse protocols aim to disrupt the fundamental metabolic processes that cancer cells depend on for survival.
Question 4: What role do cancer stem cells play in tumor development and treatment resistance?
Answer: Cancer stem cells (CSCs) function as tumor-initiating cells capable of self-renewal and differentiation, serving as the primary drivers of tumor growth and progression. These cells possess unique metabolic characteristics that allow them to survive traditional treatments and regenerate tumors, making them crucial targets for effective cancer therapy.
Treatment resistance often develops because CSCs can enter a quiescent state, making them less vulnerable to conventional therapies that target rapidly dividing cells. Additionally, these cells express specific surface markers and maintain active DNA repair mechanisms, allowing them to survive and potentially initiate tumor recurrence even after apparently successful treatment.
Question 5: How has Otto Warburg's research influenced modern understanding of cancer metabolism?
Answer: Otto Warburg's pioneering research in the 1920s fundamentally changed our understanding of cancer by identifying the distinctive metabolic pattern of cancer cells, which prefer glucose fermentation even in the presence of oxygen. His work laid the foundation for metabolic approaches to cancer treatment and continues to influence modern research into cancer cell energy metabolism.
The impact of Warburg's discoveries extends beyond his initial findings, inspiring contemporary researchers to investigate metabolic targeting as a therapeutic strategy. His observations have led to the development of various diagnostic tools, including PET scans that exploit cancer cells' high glucose uptake, and have influenced the development of metabolic therapies targeting cancer's unique energy requirements.
Question 6: What evidence supports treating cancer as a metabolic disease rather than a genetic one?
Answer: Multiple studies have demonstrated that transplanting healthy nuclei into cancer cell cytoplasm fails to reverse the cancerous state, while transferring cancer cell nuclei into healthy cytoplasm results in normal cell development. This experimental evidence strongly suggests that the cytoplasmic environment, particularly mitochondrial function, plays a more crucial role in cancer development than nuclear DNA mutations.
Clinical observations also show that many cancer-associated mutations appear in healthy tissues without causing cancer, while some cancers develop without typical oncogenic mutations. Furthermore, the success of metabolic interventions in treating various cancers, combined with the universal metabolic characteristics shared across different cancer types, supports the metabolic paradigm.
Question 7: How do oxidative phosphorylation and glycolysis differ in normal cells versus cancer cells?
Answer: Normal cells primarily rely on oxidative phosphorylation for energy production, which generates 36 ATP molecules per glucose molecule through an efficient oxygen-dependent process in the mitochondria. This process involves the complete breakdown of glucose through the citric acid cycle and electron transport chain, providing optimal energy yield for cellular functions.
Cancer cells, conversely, predominantly use glycolysis even in the presence of oxygen, producing only 2 ATP molecules per glucose molecule but generating metabolic intermediates necessary for rapid cell division. This inefficient process requires cancer cells to consume significantly more glucose than normal cells, leading to the characteristic high glucose uptake observed in tumors.
Question 8: What are the key differences between traditional cancer treatments and metabolic therapy approaches?
Answer: Traditional cancer treatments primarily target rapidly dividing cells through cytotoxic mechanisms, often resulting in significant collateral damage to healthy tissues. These approaches, including chemotherapy and radiation, focus on killing cancer cells directly without necessarily addressing the underlying metabolic conditions that support their growth.
Metabolic therapy approaches, by contrast, aim to exploit the unique metabolic vulnerabilities of cancer cells while supporting healthy cell function. These treatments often combine dietary modifications, targeted supplements, and specific drug protocols designed to disrupt cancer cell metabolism while enhancing normal cellular energy production, potentially offering fewer side effects and better long-term outcomes.
Question 9: How does cellular respiration dysfunction contribute to cancer development?
Answer: Cellular respiration dysfunction typically begins with damage to the mitochondria's ability to effectively produce energy through oxidative phosphorylation. This damage forces cells to rely more heavily on fermentation for energy production, leading to changes in cellular metabolism that can promote cancer development and progression.
The shift away from efficient oxidative phosphorylation creates a cascade of effects, including altered gene expression, increased reactive oxygen species production, and changes in cellular signaling pathways. These changes can create an environment that supports cancer cell survival and proliferation while compromising normal cellular regulatory mechanisms.
Question 10: What impact has The Cancer Genome Atlas Program had on cancer research and treatment?
Answer: The Cancer Genome Atlas Program has generated comprehensive genomic profiles of thousands of cancer samples across multiple cancer types, providing unprecedented insight into the genetic alterations associated with different cancers. This massive database has enabled researchers to identify common mutation patterns and potential therapeutic targets, leading to the development of more targeted treatment approaches.
However, the program has also revealed unexpected complexity in cancer genetics, showing that many cancers lack clear driver mutations and that genetic profiles can vary significantly even within the same tumor type. This complexity has challenged the traditional mutation-centric view of cancer and highlighted the need for more comprehensive approaches to cancer treatment.
Question 11: How do clinical trial regulations affect the development of new cancer treatments?
Answer: Clinical trial regulations create a structured framework that requires extensive safety and efficacy testing before new treatments can reach patients. This process typically involves multiple phases, starting with safety studies in small groups and progressing to larger efficacy trials, often taking 7-10 years and costing hundreds of millions of dollars to complete.
These regulations, while ensuring patient safety, can also slow the implementation of promising treatments and limit the testing of combination therapies. The stringent requirements particularly impact the development of metabolic therapies and repurposed drugs, which often lack the financial backing needed to navigate the expensive approval process.
Question 12: What role does the FDA play in cancer treatment approval and regulation?
Answer: The FDA serves as the primary gatekeeper for cancer treatment approval in the United States, establishing standards for safety and efficacy through a multi-phase review process. This agency evaluates all clinical trial data, manufacturing processes, and safety protocols before granting approval for new cancer treatments, while also monitoring post-market safety and effectiveness.
Beyond initial approval, the FDA continues to oversee treatment modifications, manufacturing changes, and safety monitoring through various reporting requirements and inspection programs. The agency's decisions significantly influence treatment availability, research direction, and pharmaceutical company investments in cancer drug development.
Question 13: How do treatment costs affect cancer care accessibility and outcomes?
Answer: Treatment costs create significant barriers to cancer care access, with many patients facing financial toxicity that can lead to bankruptcy, treatment delays, or discontinuation. The high costs of newer targeted therapies and immunotherapies, which can exceed $100,000 per year, often force patients to make difficult choices between financial stability and optimal treatment options.
Insurance coverage limitations, high deductibles, and co-payments further compound the accessibility problem, creating disparities in treatment outcomes based on socioeconomic status. These financial barriers often result in patients choosing less expensive but potentially less effective treatments, or foregoing recommended follow-up care and monitoring.
Question 14: What is the significance of tumor heterogeneity in cancer treatment?
Answer: Tumor heterogeneity refers to the presence of distinct cell populations within a single tumor, each potentially having different genetic profiles, metabolic characteristics, and treatment sensitivities. This diversity within tumors explains why single-target treatments often fail to achieve complete responses and why cancer frequently develops resistance to initially effective treatments.
The complex nature of tumor heterogeneity necessitates multi-targeted treatment approaches that can address various cell populations simultaneously. Understanding this heterogeneity has led to the development of combination therapies and personalized treatment strategies that target multiple aspects of tumor biology.
Question 15: How do metastasis mechanisms work and why are they important in cancer progression?
Answer: Metastasis involves a complex series of steps where cancer cells detach from the primary tumor, enter the bloodstream or lymphatic system, and establish new tumors in distant locations. This process requires cells to undergo significant changes in their adhesion properties, survive in circulation, and adapt to new tissue environments.
The ability to metastasize represents the most dangerous aspect of cancer, accounting for approximately 90% of cancer-related deaths. Understanding metastatic mechanisms is crucial because cancer cells that successfully metastasize often develop resistance to treatments that were effective against the primary tumor, requiring different therapeutic approaches.
Question 16: What evidence hierarchies are used to evaluate cancer research?
Answer: Evidence hierarchies in cancer research place randomized controlled trials (RCTs) and systematic reviews at the top, followed by cohort studies, case-control studies, and case reports in descending order of reliability. This structure helps clinicians and researchers evaluate the strength of evidence supporting different treatment approaches and research findings.
The hierarchy also considers factors such as study design quality, sample size, and replication of results across multiple studies. Meta-analyses combining data from multiple high-quality studies provide particularly strong evidence, though real-world evidence and observational studies are gaining recognition for their ability to reveal treatment effects in diverse patient populations.
Question 17: How do repurposed drugs fit into cancer treatment strategies?
Answer: Repurposed drugs, medications originally approved for other conditions, offer potentially effective cancer treatments at lower costs and with well-understood safety profiles. These medications often target metabolic pathways or cellular mechanisms that are relevant to both their original indication and cancer treatment, making them valuable additions to treatment protocols.
The main advantages of drug repurposing include reduced development costs, shorter time to clinical implementation, and extensive safety data from years of clinical use. However, these drugs often face challenges in gaining official approval for cancer treatment due to limited financial incentives for pharmaceutical companies to fund the necessary clinical trials.
Question 18: What factors influence the success of immunotherapy treatments?
Answer: Immunotherapy success depends heavily on the patient's immune system status, tumor type, and specific molecular markers present on cancer cells. Factors such as tumor mutational burden, PD-L1 expression levels, and microsatellite instability status significantly influence treatment response rates.
Environmental factors, including the patient's microbiome composition, overall health status, and previous treatments, also play crucial roles in determining immunotherapy outcomes. The timing of immunotherapy administration and its combination with other treatment modalities can significantly impact its effectiveness.
Question 19: How does genomic instability contribute to cancer development?
Answer: Genomic instability creates an environment where DNA damage accumulates more rapidly than in normal cells, leading to increased mutation rates and chromosomal abnormalities. This process accelerates the acquisition of cancer-promoting mutations and helps explain the progressive nature of cancer development.
The relationship between genomic instability and metabolic dysfunction appears bidirectional, with metabolic stress contributing to DNA damage and repair deficiencies, while genetic alterations can further disrupt normal cellular metabolism. This complex interaction creates a self-reinforcing cycle that promotes cancer progression and treatment resistance.
Question 20: What role do environmental factors play in cancer risk and prevention?
Answer: Environmental factors significantly influence cancer risk through various mechanisms, including direct DNA damage, epigenetic modifications, and alterations in cellular metabolism. These factors encompass a broad range of exposures, from dietary choices and physical activity levels to chemical exposures and radiation.
The impact of environmental factors varies depending on individual genetic susceptibility, timing of exposure, and duration of exposure. Understanding these relationships has led to the development of prevention strategies that focus on modifiable risk factors, though the complex interactions between environmental exposures and biological responses often make it difficult to establish direct causal relationships.
Question 21: How do hormonal factors influence cancer development and progression?
Answer: Hormones act as powerful regulators of cell growth, differentiation, and metabolism, directly influencing cancer risk and progression through multiple pathways. Estrogen, testosterone, insulin, and growth hormones can promote cell proliferation, alter gene expression, and influence cellular energy metabolism in ways that support cancer development and growth.
The timing of hormonal exposure throughout life plays a crucial role, with periods such as puberty, pregnancy, and menopause representing particularly sensitive windows. This understanding has led to the development of hormone-targeted therapies and preventive strategies, including the use of selective hormone receptor modulators in breast and prostate cancers.
Question 22: What are the primary differences between observational studies and clinical trials in cancer research?
Answer: Observational studies examine cancer patterns and outcomes in real-world settings without intervention, allowing researchers to identify associations between various factors and cancer development or progression. These studies often provide valuable insights into long-term outcomes, rare side effects, and treatment effectiveness in diverse populations not typically included in clinical trials.
Clinical trials, conversely, involve controlled interventions where researchers actively manipulate variables to test specific hypotheses about treatment efficacy and safety. While clinical trials provide the strongest evidence for causation and treatment effectiveness, they often have stricter inclusion criteria and may not fully represent real-world patient populations.
Question 23: How does the patent system influence cancer drug development and accessibility?
Answer: The patent system provides pharmaceutical companies with temporary monopolies on new drugs, incentivizing investment in expensive research and development programs. This protection allows companies to charge premium prices during the patent period to recoup development costs and generate profits, significantly affecting drug pricing and accessibility.
However, this system also creates disincentives for developing treatments with limited patent protection, such as repurposed drugs or natural compounds. The focus on patentable innovations often directs research away from potentially effective but less profitable treatment approaches, influencing both the types of treatments developed and their eventual cost to patients.
Question 24: What role do meta-analyses play in determining cancer treatment efficacy?
Answer: Meta-analyses combine and statistically analyze results from multiple studies, providing a comprehensive view of treatment effectiveness across different patient populations and research settings. This approach increases statistical power and can reveal patterns or effects that might not be apparent in individual studies, particularly for treatments with modest benefits or specific subgroup effects.
The systematic nature of meta-analyses helps identify potential biases, inconsistencies, and gaps in current research while providing more precise estimates of treatment effects. These studies often inform clinical guidelines and treatment recommendations, though their validity depends heavily on the quality of included studies and the appropriateness of statistical methods used.
Question 25: How do quality of life measures impact cancer treatment decisions?
Answer: Quality of life measures provide crucial information about treatment impacts beyond survival statistics, including physical symptoms, emotional well-being, and functional capacity. These measures help patients and clinicians make informed decisions by balancing potential survival benefits against treatment side effects and their impact on daily life.
The integration of quality of life assessments into treatment planning has led to more patient-centered approaches, including the development of less toxic treatments and better supportive care strategies. These measures also help identify areas where additional support or intervention might improve treatment outcomes and patient satisfaction.
Question 26: What is the relationship between mother's age at first birth and cancer risk?
Answer: Maternal age at first birth influences cancer risk through complex hormonal and cellular mechanisms that affect tissue development and long-term cancer susceptibility. Women who have their first child at a younger age generally experience reduced breast cancer risk due to early differentiation of breast tissue and changes in hormonal profiles that create a less cancer-promoting environment.
The protective effect appears to be related to the timing of cellular differentiation in hormone-sensitive tissues and the modification of stem cell populations. However, this relationship varies by cancer type and is influenced by other reproductive factors such as total number of pregnancies, breastfeeding duration, and overall hormonal exposure throughout life.
Question 27: How do caesarean births potentially influence cancer risk in offspring?
Answer: Caesarean deliveries affect offspring cancer risk through alterations in early microbiome development and immune system programming. Babies born via C-section miss exposure to beneficial maternal vaginal and gut bacteria during birth, potentially leading to differences in immune system development and metabolic programming.
These early life differences may influence long-term cancer susceptibility through altered inflammatory responses and metabolic regulation. Research indicates that C-section delivery is associated with slight increases in certain childhood cancers, though the mechanisms are still being investigated and may involve multiple factors including surgical stress and antibiotic exposure.
Question 28: What role does family size play in cancer epidemiology?
Answer: Family size influences cancer risk through various biological and environmental pathways, including changes in immune system development and exposure patterns. Larger families often show different patterns of childhood infection exposure, which can affect immune system development and subsequent cancer risk later in life.
The relationship between family size and cancer risk also involves socioeconomic factors, lifestyle patterns, and reproductive behaviors. These associations vary by cancer type and are often modified by factors such as birth order, spacing between siblings, and shared environmental exposures within the family unit.
Question 29: How do international research standards affect global cancer treatment development?
Answer: International research standards ensure consistency and quality in cancer research across different countries and institutions, facilitating the comparison and combination of results from multiple studies. These standards establish common protocols for conducting clinical trials, reporting results, and protecting patient rights, enabling collaborative research efforts and faster development of new treatments.
However, variations in regulatory requirements and research infrastructure between countries can create challenges in implementing global studies and translating findings into practice. These differences can affect everything from trial design to patient recruitment, potentially limiting the generalizability of results and creating disparities in access to new treatments.
Question 30: What is the significance of apoptosis in cancer treatment?
Answer: Apoptosis, or programmed cell death, represents a crucial target in cancer treatment as cancer cells often develop mechanisms to evade this natural process. Effective cancer treatments must often overcome these survival mechanisms to trigger cell death in cancer cells while sparing healthy tissue.
Understanding the molecular pathways involved in apoptosis has led to the development of targeted therapies that specifically activate death signals in cancer cells. The success of these treatments often depends on their ability to overcome multiple anti-apoptotic mechanisms that cancer cells develop, requiring combination approaches that target different aspects of cell death regulation.
Question 31: How do cell signaling pathways contribute to cancer development?
Answer: Cell signaling pathways form complex networks that regulate cell growth, survival, and metabolism, with disruptions in these pathways often leading to cancer development. Aberrant signaling can result from mutations, environmental factors, or metabolic changes, creating self-sustaining loops that promote continuous cell growth and survival even in unfavorable conditions.
These pathways often become interconnected in cancer cells, creating redundant survival mechanisms that make treatment more challenging. Understanding these signaling networks has led to the development of targeted therapies that attempt to block specific pathways, though cancer cells frequently develop alternative signaling routes to maintain their growth and survival advantages.
Question 32: What role does the European Medicines Agency play in cancer treatment approval?
Answer: The European Medicines Agency (EMA) evaluates and monitors cancer treatments across the European Union, providing a centralized approval process that influences global drug development and availability. The agency's decisions affect treatment access for millions of patients and help establish international standards for drug safety and efficacy.
The EMA's approach often differs from other regulatory bodies in its evaluation criteria and approval processes, sometimes leading to different treatment availability between regions. These differences can provide valuable comparative data on treatment outcomes and safety profiles across different populations and healthcare systems.
Question 33: How do insurance coverage policies affect cancer treatment options?
Answer: Insurance coverage policies directly influence treatment decisions through formulary restrictions, prior authorization requirements, and cost-sharing structures. These policies can limit access to certain treatments or force patients to choose between therapeutic options based on financial rather than medical considerations.
The complexity of insurance coverage often creates disparities in treatment access, with some patients receiving optimal care while others face significant barriers to recommended treatments. This situation particularly affects access to newer therapies, clinical trials, and supportive care services that might improve treatment outcomes.
Question 34: What are the key factors in clinical trial funding and design?
Answer: Clinical trial funding sources significantly influence study design, treatment selection, and endpoint determination. Commercial funding typically focuses on patentable treatments with potential market value, while public or academic funding might pursue broader research questions or investigate less profitable but potentially valuable therapeutic approaches.
Trial design must balance scientific rigor with practical considerations such as patient recruitment, cost constraints, and regulatory requirements. The choice of endpoints, control groups, and inclusion criteria can significantly impact both the scientific validity of results and their practical applicability to real-world treatment decisions.
Question 35: How do standard of care protocols evolve in cancer treatment?
Answer: Standard of care protocols develop through a complex process involving clinical trial results, real-world evidence, expert consensus, and practical considerations. These protocols typically change incrementally as new evidence emerges, though occasionally dramatic breakthroughs can lead to rapid shifts in treatment approaches.
The evolution of care standards must balance the integration of new treatments with practical considerations such as cost, availability, and implementation challenges. This process often varies by region and healthcare system, leading to differences in standard treatments across different countries and practice settings.
Question 36: What impact does tumor microenvironment have on treatment effectiveness?
Answer: The tumor microenvironment creates a complex ecosystem that significantly influences treatment response through factors such as blood vessel formation, immune cell infiltration, and metabolic interactions. This environment can protect cancer cells from therapeutic agents by creating physical barriers, altering drug metabolism, and promoting resistance mechanisms.
The dynamic nature of the tumor microenvironment means it can adapt to treatments, often developing protective mechanisms that reduce therapeutic effectiveness over time. Understanding these adaptations has led to strategies targeting both cancer cells and their supporting environment, including approaches to modify blood vessel formation, immune response, and metabolic interactions.
Question 37: How do survival rates vary across different cancer types and stages?
Answer: Survival rates show marked variation depending on cancer type, stage at diagnosis, and molecular characteristics of the tumor. While some cancers now have high cure rates when caught early, others remain challenging to treat effectively even at initial stages, reflecting fundamental differences in tumor biology and treatment responsiveness.
Stage-specific survival rates provide important prognostic information but must be interpreted within the context of individual patient factors such as age, overall health status, and specific tumor characteristics. These rates continue to evolve as new treatments become available and early detection methods improve.
Question 38: What role does inflammation play in cancer development and progression?
Answer: Chronic inflammation creates conditions that promote cancer development through multiple mechanisms, including DNA damage, increased cell proliferation, and altered tissue metabolism. This inflammatory environment can support the initial transformation of normal cells into cancer cells and continue to promote tumor growth and spread.
The relationship between inflammation and cancer involves complex interactions between immune cells, signaling molecules, and metabolic processes. Understanding these relationships has led to therapeutic strategies targeting inflammatory pathways, though the challenge lies in distinguishing between harmful and beneficial inflammatory responses.
Question 39: How do side effect frequencies influence treatment selection?
Answer: Side effect profiles significantly influence treatment selection by affecting both quality of life and the ability to maintain therapeutic dosing. The frequency and severity of side effects must be balanced against potential benefits, considering factors such as patient age, overall health status, and treatment goals.
Treatment selection often involves comparing different side effect profiles across available options, with consideration for both short-term toxicities and potential long-term complications. This assessment becomes particularly important in situations where multiple treatment options offer similar efficacy but different side effect patterns.
Question 40: What is the significance of biomarker testing in cancer diagnosis and treatment?
Answer: Biomarker testing provides crucial information about tumor characteristics that can predict treatment response and guide therapy selection. These molecular indicators help identify patients most likely to benefit from specific treatments, allowing for more personalized therapeutic approaches and potentially better outcomes.
The evolution of biomarker testing has led to increasingly sophisticated treatment selection processes, though challenges remain in determining which markers are most relevant for different situations. The cost and availability of testing can also create barriers to optimal treatment selection, particularly in resource-limited settings.
Question 41: How does drug resistance develop in cancer cells?
Answer: Drug resistance develops through multiple adaptive mechanisms, including increased drug efflux, altered drug targets, enhanced DNA repair, and metabolic rewiring. Cancer cells can acquire these resistance mechanisms either through genetic mutations or through rapid adaptation of cellular processes, often involving multiple pathways simultaneously.
The development of resistance frequently involves the selection of pre-existing resistant cell populations within heterogeneous tumors, followed by their expansion under treatment pressure. This process is complicated by the ability of cancer cells to share resistance mechanisms through intercellular communication and the release of protective factors into the tumor microenvironment.
Question 42: What role does epigenetics play in cancer development?
Answer: Epigenetic modifications influence cancer development by altering gene expression patterns without changing DNA sequences, affecting cellular differentiation, metabolism, and growth control. These changes can be inherited through cell division and may be influenced by environmental factors, creating a link between external conditions and cancer risk.
The reversible nature of epigenetic modifications makes them potential therapeutic targets, leading to the development of treatments that aim to restore normal gene expression patterns. Understanding epigenetic mechanisms has revealed how early life exposures and lifestyle factors can influence cancer risk through persistent changes in gene regulation.
Question 43: How do different imaging technologies contribute to cancer diagnosis and monitoring?
Answer: Modern imaging technologies provide crucial information about tumor location, size, metabolic activity, and response to treatment through various modalities including PET, CT, MRI, and molecular imaging. Each technology offers unique advantages in visualizing different aspects of cancer biology, allowing for comprehensive assessment of disease status and progression.
The integration of multiple imaging approaches, often combined with artificial intelligence analysis, enables more accurate diagnosis, staging, and treatment monitoring. These technologies continue to evolve, offering increasing precision in detecting small tumors, assessing treatment response, and guiding surgical interventions.
Question 44: What is the importance of early detection in cancer treatment outcomes?
Answer: Early detection significantly improves treatment outcomes by identifying cancers before they become extensively established or metastatic, allowing for more targeted and less aggressive interventions. When detected early, many cancers can be treated with curative intent, often requiring less extensive surgery and fewer systemic treatments.
The benefits of early detection must be balanced against the risks of overdiagnosis and unnecessary treatment, particularly in screening programs. This balance varies by cancer type and screening method, requiring careful consideration of both individual risk factors and population-level benefits.
Question 45: How do dietary factors influence cancer risk and treatment outcomes?
Answer: Dietary factors affect cancer risk through multiple mechanisms, including influence on inflammation, hormone levels, and cellular metabolism. Specific dietary patterns can either promote or inhibit cancer development through their effects on oxidative stress, DNA repair, and immune function.
During cancer treatment, dietary choices can influence treatment effectiveness and side effect management. The metabolic requirements of cancer cells and their response to nutrient availability have led to increasing interest in dietary interventions as part of comprehensive treatment strategies, though optimal approaches often vary by cancer type and treatment protocol.
Question 46: What role does the immune system play in cancer prevention and treatment?
Answer: The immune system serves as a critical defense against cancer development through continuous surveillance and elimination of potentially cancerous cells. This natural protection involves multiple immune cell types working together to recognize and destroy abnormal cells before they can establish tumors, while also maintaining memory against cancer-specific antigens.
Immunotherapy treatments aim to enhance or restore this natural anti-cancer immunity through various approaches, including checkpoint inhibition, CAR-T cell therapy, and cancer vaccines. The success of these treatments depends heavily on understanding the complex interactions between immune cells and cancer cells, as well as the mechanisms tumors use to evade immune recognition.
Question 47: How do targeted therapies differ from traditional chemotherapy?
Answer: Targeted therapies are designed to interfere with specific molecular pathways essential for cancer cell growth and survival, unlike traditional chemotherapy which affects all rapidly dividing cells. These treatments often focus on unique characteristics of cancer cells, such as specific protein mutations or overexpressed growth factors, potentially offering greater effectiveness with fewer side effects.
The specificity of targeted therapies requires detailed molecular profiling of tumors to identify appropriate treatment targets, leading to more personalized treatment approaches. However, cancer cells often develop resistance to targeted therapies through alternative pathways, necessitating combination approaches or sequential treatment strategies.
Question 48: What is the significance of personalized medicine in cancer treatment?
Answer: Personalized medicine in cancer treatment involves tailoring therapeutic approaches based on the specific molecular characteristics of individual tumors and patient factors. This approach integrates genetic profiling, biomarker testing, and clinical characteristics to select the most appropriate treatments for each patient, potentially improving outcomes while minimizing unnecessary treatments.
The implementation of personalized medicine requires sophisticated diagnostic testing and data analysis capabilities, creating both opportunities and challenges for healthcare systems. This approach continues to evolve as new technologies and treatments become available, though cost and accessibility remain significant considerations.
Question 49: How do radiation therapy mechanisms affect both cancer and healthy cells?
Answer: Radiation therapy works by causing DNA damage that leads to cell death, with cancer cells being generally more susceptible due to their compromised repair mechanisms and rapid division rates. The biological effects of radiation extend beyond direct DNA damage to include changes in the tumor microenvironment and systemic immune responses.
Modern radiation techniques aim to maximize damage to tumor tissue while sparing healthy cells through precise targeting and fractionation schedules. The development of new delivery methods and combinations with other treatments continues to improve the therapeutic window of radiation therapy.
Question 50: What role does stress play in cancer development and progression?
Answer: Chronic stress influences cancer development and progression through multiple biological pathways, including effects on immune function, inflammation, and hormone levels. Stress-related changes in the body can create conditions that promote cancer cell survival and spread while potentially reducing the effectiveness of natural anti-cancer mechanisms.
The relationship between stress and cancer involves both direct biological effects and indirect influences through behavior changes and treatment adherence. Understanding these connections has led to increased attention to stress management as part of comprehensive cancer care, though measuring and modifying stress effects remains challenging.
I appreciate you being here.
If you've found the content interesting, useful and maybe even helpful, please consider supporting it through a small paid subscription. While everything here is free, your paid subscription is important as it helps in covering some of the operational costs and supports the continuation of this independent research and journalism work. It also helps keep it free for those that cannot afford to pay.
Please make full use of the Free Libraries.
Unbekoming Interview Library: Great interviews across a spectrum of important topics.
Unbekoming Book Summary Library: Concise summaries of important books.
Stories
I'm always in search of good stories, people with valuable expertise and helpful books. Please don't hesitate to get in touch at unbekoming@outlook.com
For COVID vaccine injury
Consider the FLCCC Post-Vaccine Treatment as a resource.
Baseline Human Health
Watch and share this profound 21-minute video to understand and appreciate what health looks like without vaccination.





Amazing article. However, until/unless we address the CAUSES of cancer, this information is only partly the solution.
As someone who has had cancer twice and one of 7 women in my family who've been diagnosed, I'm angry about the lack of inquiry regarding the root environmental issues that triggers all these cancers.
The conventional treatment killed my younger sister who had the same diagnosis as me at the same time as me twice, in 2012 and 2015. I chose not to do the chemo/radiation (used holistic alternative healing modalities instead as recommended by a naturopath), she was forced to by her employer against her wishes because she would not receive disability income otherwise.
Like so many others, I believe she was murdered by the medical system. But more importantly, I believe we're all being poisoned by corporations who profit at our expense. Not to mention all the charities who think they're helping cancer patients. It's all a racket.
All disease comes from Vaccines, end of story. Second in command, the food supply. Let the shit rot on the shelves and buy organic. Reverse osmosis your water. Get in the sun, take Vit D. There's a reason it's called the cancer vitamin. Intermittent fasting. Open your windows even on the coldest of days. Let shit go and laugh your ass off whenever possible. Never do wellness checks. Stay away from Allopaths. Your cancer risk will drop to zippo.