Thimerosal (2015)
By Robert F. Kennedy Jr. – 50 Q&As – Unbekoming Book Summary
The first time I heard Bobby speak was when he was promoting Thimerosal on the Bill Maher show. This must have been about 10 years ago, back in my “centre left” phase.
I remember telling my wife afterwards: “I really like Maher, but why does he keep pushing this anti vax nonsense.”
This is straight after Bobby had just told me that I had injected mercury into my kids.
When you’re brainwashed, you’re brainwashed.
Bobby gets full credit for forcing them to remove “the preservative” mercury from almost all injections, but it remains in flu shots, nourishing the Alzheimer’s Cartel.
Its removal has not made a dent in the autism trend, I suspect because they have simply added more aluminum into the total load by increasing the number of injections. If they hadn’t have done that, while removing the mercury, you would have likely seen a drop in autism rates. It would have stuck out like a sore thumb, and that is certainly not acceptable.
If you are new to this subject, it just doesn’t make sense that they would knowingly put a neurotoxin into everybody. That sounds like crazy talk. The problem though is that when you are new to this subject, you, like I used to, have the wrong model of reality. That’s why it doesn’t make sense.
The longer you spend in this subject, and if your personality can stomach the conclusion, you eventually land on a more accurate model of reality, as expressed here by Jason Christoff.
Mass poisoning and mass psychosis (mind control) are the two most effective means of “managing” society, the mass.
What makes sense to the cattle rancher would not make sense to the cattle.
With thanks to Robert Kennedy Jr.
Related Posts
Deep Dive Conversation Library (Bonus for Paid Subscribers)
This deep dive is based on the book:
Discussion No.36:
20 important insights from “Thimerosal”
Thank you for your support.
Analogy
Imagine a construction company discovers that a widely-used concrete additive, while making production cheaper and easier, contains a potent toxin. Early safety studies were conducted on buildings that collapsed too quickly to assess long-term structural damage. When concerning cracks start appearing in buildings years later, particularly in children's schools, the company points to studies showing the additive clears quickly from the surface - while ignoring evidence it's actually accumulating in the foundations.
Despite some countries banning the additive and safer alternatives existing, the company continues using it in certain buildings, arguing it's too expensive to change. They cite studies from countries that used much less of the additive, and where record-keeping changed significantly, to claim there's no proof of harm. Meanwhile, their own internal documents show early data revealed serious problems, but the research was repeatedly revised until the connection disappeared.
The company acknowledges the additive is so toxic that leftover materials must be handled as hazardous waste, yet maintains it's perfectly safe in their buildings. When independent engineers try to examine the original data, they're denied access. The company insists that examining alternatives would waste resources better spent studying why buildings are cracking.
This analogy mirrors how a known neurotoxin remained in vaccines despite flawed safety studies, evidence of harm, existing alternatives, and the paradox of being considered too dangerous for topical use or disposal while supposedly safe for injection into infants and pregnant women. It highlights how economic arguments and compromised research were used to maintain a dangerous status quo, even as evidence mounted suggesting the need for change.
12-point summary
Chemical Composition and Basic Facts: Thimerosal is a mercury-containing compound (49.55% mercury by weight) used as a vaccine preservative since the 1930s. Initial safety studies were fundamentally flawed, with the primary study involving 22 meningitis patients who all died shortly after administration.
Exposure History: Children's mercury exposure from vaccines more than doubled between 1989 and 1999, from 100 to 237.5 micrograms by age two. This increase coincided with a dramatic rise in neurodevelopmental disorders, including autism rates rising from approximately 1 in 2,000 to as high as 1 in 166.
Mercury Toxicity Mechanism: Mercury disrupts critical cellular processes, including methylation and DNA repair. Studies show it can be toxic to developing human neurons at concentrations as low as 4.35 nanomolar - below the conventional safety threshold of 29 nanomolar in umbilical cord blood.
Ethylmercury vs. Methylmercury: While ethylmercury clears blood faster than methylmercury, research shows it accumulates more readily in the brain, where it converts to inorganic mercury with a half-life of up to 540 days. Studies suggest ethylmercury may be more toxic than methylmercury.
Aluminum Interaction: Research demonstrates dangerous synergistic effects between Thimerosal and aluminum adjuvants in vaccines. Combined exposure killed approximately 90% of neurons in culture over 24 hours, compared to 65% with Thimerosal alone and 10% with aluminum alone.
Pregnancy Risks: Thimerosal readily crosses the placental barrier. Even assuming 99% placental blocking, a single Thimerosal-preserved flu shot exposes a fetus to unsafe mercury levels throughout most of gestation. At 90% blocking, exposure reaches 100 times the EPA's safe reference dose.
The Verstraeten Study Evolution: The CDC's flagship safety study underwent at least six revisions. Initial analysis showed children receiving highest mercury doses were 7.6 times more likely to develop autism. Final published version in 2003 found no consistent significant associations.
European Studies Limitations: Studies from Denmark, Sweden and the UK involved significantly lower mercury exposures than US children received. Changes in diagnostic criteria and reporting methods artificially inflated autism rates after Thimerosal's removal, compromising these studies' conclusions.
Alternative Options: 2-phenoxyethanol (2-PE) has been shown to be 70 times less toxic than Thimerosal while providing better antimicrobial protection. Single-dose vials eliminate preservative needs entirely, though at higher manufacturing costs.
Economic Considerations: While switching to single-dose vials globally would cost approximately $300 million annually, this compares to autism costs in the US alone of $137 billion per year. Studies show 60% wastage in multi-dose vials offsets their apparent cost advantages.
Scientific Review Problems: The 2004 IOM report relied almost exclusively on epidemiological studies while dismissing biological and toxicological research. The committee focused narrowly on autism rather than considering broader neurodevelopmental effects.
Current Status: Despite mounting evidence and safer alternatives, Thimerosal remains in multi-dose flu vaccines given to pregnant women and children. About 39% of flu vaccine doses in 2013-2014 contained Thimerosal, while paradoxically these same vaccines must be disposed of as hazardous waste due to their mercury content.
Preface
Vaccinations are among the most important advances in medicine in the last century. We have eradicated smallpox from the planet and dramatically reduced death and suffering from infectious disease around the globe. I am aggressively pro-vaccine, as are the editor and introduction contributor of this book. I am a father and family physician. I have vaccinated my children. I have been vaccinated and recommend vaccination to my patients.
Critics of this book will quickly polarize the debate. It is easy to oversimplify the issue of Thimerosal into pro-vaccine or anti-vaccine, or to confuse this issue by debating whether Thimerosal causes autism, which has not been definitively proven. This is unfortunate, and detracts from a much simpler set of questions that are ultimately the subject of this scientifically dense book.
There is no debate that mercury in any form is toxic. Scientists may debate the differences in toxicity between different forms of mercury, such as ethylmercury (which is an ingredient in Thimerosal) or methylmercury (from fish). But all would agree that mercury is a potent neurotoxin.
There is also no debate about the dramatic increase in prevalence of neurodevelopmental disorders over the last few decades, including learning disabilities, attention deficit disorders, and autism.
There may, however, be debate on the strength of the data and science implicating mercury in this increased prevalence of brain injury in children. These questions can never be adequately answered given the challenges of doing experimental studies on human subjects over long periods of time. Obviously, no ethical review board would ever approve a study in which children were purposefully exposed to mercury in order to test its toxicity. Population studies show correlations, but never prove causation, making it impossible to draw firm conclusions.
That leaves us with a very simple, moral question, and ultimately a very personal one. Because at some point in our lives, nearly all of us will have a child or grandchild who requires vaccinations. Or we will know a pregnant woman who will have to decide whether or not to get a flu shot that might contain mercury. All of us are people and parents first, and scientists and policy makers second.
So there is only one question that really matters:
Would you expose the unborn child or infant of a loved one to a vaccine containing mercury, a known neurotoxin, if there were other safer alternatives?
The answer to this question is simply common sense and requires no further scientific inquiry, but as Voltaire said, “common sense is not so common.” If there were no other options, if it were a question of whether to vaccinate or not to vaccinate, then of course we would choose vaccination. But that is a false choice. There are 137 million children born each year in the world. Is our only option to subject them to a potent neurotoxin in their most delicate neurodevelopmental period? How can we best protect that future generation from preventable harm?
The arguments put forth that we cannot remove Thimerosal from vaccines are invalid. Thimerosal has already been removed from nearly all vaccines except the multidose flu vaccine in the United States. This was based on government recommendations and a call to action from many agencies and health organizations, as is well documented in this book. However, Thimerosal still remains in nearly all the pediatric vaccines used in the developing world. There are effective alternative preservatives already in use (2-phenoxyethanol), and new ones can be developed. The Food and Drug Administration (FDA) banned mercury as a topical antiseptic (remember Mercurochrome?). And any medical products containing Thimerosal or mercury cannot be thrown in the garbage. The Environmental Protection Agency (EPA) considers them hazardous waste.
Does it make any sense that even though Thimerosal is not safe to put on your skin, or to throw in the garbage, it is safe to inject into pregnant women and babies?
Cost considerations are also used as an argument to keep Thimerosal in vaccines. There is a small cost increase to use single-dose flu vaccines, but it is minor compared to the cost of neurodevelopmental disease in children. The global cost of taking Thimerosal out of all vaccines is $300 million a year, while the annual cost of autism in the United States alone is well over $100 billion. In the developing world, studies show that there is significant wastage of multidose vials, making single-dose vials comparable in cost.
There are other arguments. Some scientists we spoke to at the Department of Health and Human Services said that Thimerosal may contribute to the effectiveness of the vaccines. Any agent that increases vaccine effectiveness is referred to as an adjuvant. However, Thimerosal is approved for use only as a preservative, not as an active ingredient, and such use is illegal.
I have been involved in reviewing and contributing ideas and scientific references to this manuscript. I have also been involved in efforts to change regulatory and legislative policy to reduce potential harm from Thimerosal. I do not belong to any organization connected in any way with this issue. Nor do I have any personal or financial interest in this issue other than a scientific and moral one.
And, as a physician, my Hippocratic oath is to “first, do no harm.” We should practice the precautionary principle in medicine and avoid doing harm whenever possible. And given the simple fact that mercury is toxic, I can come to no other conclusion than this: we should immediately remove Thimerosal from vaccines and all other products used in medicine.
Mark Hyman, MD
West Stockbridge, Massachusetts
June 7, 2014
50 Questions & Answers
Question 1: What is Thimerosal and when was it first introduced into medical products?
Thimerosal is a mercury-containing compound that consists of 49.55 percent mercury by weight in the form of ethylmercury bound to thiosalicylate. First trademarked in 1928 by pharmaceutical company Eli Lilly as Merthiolate, the white powder compound was initially used in various antiseptic products, including nasal sprays, eyewashes, vaginal spermicides, and diaper rash treatments.
In the 1930s, pharmaceutical companies began incorporating Thimerosal into vaccines as a preservative to prevent bacterial contamination. The decision to use Thimerosal was based on early studies by H.M. Powell and W.A. Jamieson in 1931, though these studies were problematic in their methodology and conclusions about safety.
Question 2: How does Thimerosal's chemical composition differ from other forms of mercury?
Thimerosal contains ethylmercury, which differs structurally from methylmercury found in seafood. While both are organic mercury compounds, ethylmercury has been historically considered less toxic because it allegedly clears from blood more quickly than methylmercury. However, this rapid clearance may actually indicate that ethylmercury moves more quickly into organs and tissues, particularly the brain.
Research has shown that ethylmercury compounds can be more toxic than methylmercury in some cases, with studies indicating that ethylmercury can be more persistent in the brain and may cause more severe cellular damage. The distinction between these forms of mercury has been used to justify Thimerosal's continued use, despite evidence suggesting ethylmercury could be equally or more dangerous than methylmercury.
Question 3: What was the original purpose of adding Thimerosal to vaccines?
Thimerosal was introduced into vaccines as a preservative to prevent bacterial contamination in multidose vials. The primary concern was that when needles were inserted multiple times into vaccine vials to withdraw doses, bacteria could be introduced and potentially grow in the solution, creating a risk of infection for vaccine recipients.
However, studies as early as 1935 showed that Thimerosal was actually 35.3 times more toxic to embryonic chicken heart tissue than to the bacteria it was meant to kill. Further research continued to demonstrate that Thimerosal was a poor choice as a preservative, being only "slowly bactericidal" and mainly "bacteriostatic," meaning it inhibited bacterial growth rather than killing bacteria outright.
Question 4: How did vaccine schedules and Thimerosal exposure change between 1970-1999?
From the early 1970s through the late 1980s, children typically received only three types of vaccinations totaling eight injections in their first eighteen months, with mercury exposure around 100 micrograms by age two. Starting in 1989, the vaccine schedule expanded considerably with the addition of Haemophilus b conjugate vaccine (Hib) and hepatitis B vaccine, among others.
By 1999, children were receiving up to nineteen vaccine injections before age two, with potential mercury exposure reaching 237.5 micrograms - more than double the previous level. This dramatic increase occurred during a critical period of neurodevelopment, with some two-month-old infants receiving doses that exceeded EPA safety guidelines by 125 times when calculated by body weight.
Question 5: What early studies were conducted to establish Thimerosal's safety?
The primary safety study used by Eli Lilly involved an experiment conducted by K.C. Smithburn in 1929 at Indianapolis City Hospital. The study administered Thimerosal to twenty-two patients suffering from meningococcal meningitis. All patients died, seven within one day of Thimerosal administration, yet the researchers declared the experiment a success because patients showed no immediate negative reactions to the preservative.
This deeply flawed study became the basis for Eli Lilly's safety claims for decades, despite the fact that the patients' existing illness could have masked any short-term effects of Thimerosal toxicity. Additionally, the brief survival period of the patients made it impossible to assess any long-term neurological effects of mercury exposure.
Question 6: How does ethylmercury differ from methylmercury in its biological effects?
Ethylmercury from Thimerosal clears from blood more rapidly than methylmercury, but this faster clearance appears to be due to its enhanced ability to cross into organs and tissues. Studies, particularly those by Thomas Burbacher in 2005, showed that ethylmercury-exposed primates retained twice the level of inorganic mercury in their brains compared to methylmercury-exposed primates, with this mercury having a half-life of up to 540 days.
Both forms of mercury are potent neurotoxins, but ethylmercury may be more dangerous due to its enhanced ability to cross the blood-brain barrier and convert to inorganic mercury, which becomes trapped in brain tissue. Studies have shown that ethylmercury compounds can be more toxic than methylmercury in various animal models, with some research indicating lower lethal doses for ethylmercury compared to methylmercury.
Question 7: What are the mechanisms by which mercury crosses the blood-brain barrier?
Mercury in both ethyl and methyl forms readily crosses the blood-brain barrier due to their lipophilic properties, but ethylmercury appears to have enhanced ability to penetrate brain tissue. The compounds disrupt the formation of microtubules in nerve cells, interfere with neurotransmitter function, and can cause direct cellular damage through oxidative stress and mitochondrial dysfunction.
Once in the brain, mercury compounds, particularly ethylmercury, can be converted to inorganic mercury which becomes trapped in brain tissue. This conversion and trapping process leads to prolonged exposure and potential chronic neurotoxic effects, even after blood levels have returned to normal. This mechanism helps explain why quick blood clearance of ethylmercury does not necessarily indicate safety.
Question 8: How do aluminum adjuvants interact with Thimerosal in vaccines?
Aluminum and Thimerosal demonstrate a synergistic toxic effect when combined in vaccines. Research by Haley in 2005 showed that when aluminum and Thimerosal were combined, they caused significantly more neuronal cell death than either compound alone. After five hours of exposure, the combination killed about half of available neurons in culture, compared to only 5 percent cell death with either compound individually.
Both aluminum and mercury share common neurotoxic properties, including interference with neurotransmitters, disruption of neuronal membranes, and increase in oxidative stress. Over twenty-four hours, the combination of both compounds resulted in approximately 90 percent neuronal cell death, compared to 65 percent with Thimerosal alone and 10 percent with aluminum alone.
Question 9: What are the documented neurological effects of mercury exposure?
Mercury exposure can severely disrupt normal neurodevelopmental processes in the human brain, causing problems in migration and division of neurons, as well as cell degeneration and death. Studies of populations exposed to mercury, such as in the Faroe Islands, have shown that prenatal exposure results in persistent cognitive deficits in language, attention, memory, and visuospatial and motor functions.
These effects appear to be irreversible and can persist into adolescence and beyond. Research has shown that mercury exposure during fetal development and early childhood can lead to persistent IQ deficits, with estimates suggesting that mercury exposure causes IQ losses ranging from three-quarters of a point to more than three points, resulting in significant economic impacts through diminished lifetime productivity.
Question 10: How does Thimerosal affect fetal development during pregnancy?
Thimerosal readily crosses the placental barrier, with studies suggesting that ethylmercury may pass even more easily than methylmercury. Research has shown that cord blood mercury concentrations can reach 4.4 times that of the mother's blood level, and animal studies indicate that ethylmercury proportionally accumulates more in fetal tissue compared to maternal tissue, particularly in the central nervous system.
A 2012 study by Brown and Austin found that even assuming the placenta blocks 99 percent of available mercury, a fetus exposed to a single Thimerosal-preserved flu shot would still receive unsafe mercury levels throughout most of gestation. Assuming 90 percent placental blocking, a fetus at the start of the second trimester would receive about one hundred times the EPA's reference dose for methylmercury.
Question 11: What evidence exists for Thimerosal's effects on infant brain development?
Studies in animal models have consistently shown that Thimerosal exposure during early development can cause significant neurological damage. Research on infant macaques by Burbacher demonstrated that Thimerosal-exposed primates retained twice the level of inorganic mercury in their brains compared to methylmercury-exposed animals, with mercury deposits persisting for extended periods. These findings were particularly significant because the doses used were within the range that children received from vaccines.
A 2000 study by Stajich found a "significant increase" in mercury levels in both preterm and term infants after receiving Thimerosal-containing vaccines, with preterm infants showing markedly higher levels. Additional research in 2005 showed that Thimerosal could be toxic to developing human neurons at concentrations as low as 4.35 nanomolar - below the 29 nanomolar concentration considered the safe limit for mercury in umbilical cord blood.
Question 12: How do mercury levels from vaccines compare to EPA safety guidelines?
A single Thimerosal-preserved flu vaccine contains 25 micrograms of ethylmercury, which would require an individual to weigh more than 250 kilograms (551 pounds) to meet the EPA's safe reference dose guidelines. For young children receiving half-doses of Thimerosal-preserved flu shots, this still represents approximately fourteen times a safe daily exposure for a 20-pound (9-kilogram) individual.
In the 1990s, a two-month-old child weighing approximately 5 kilograms (11 pounds) could receive 62.5 micrograms of mercury from three vaccines in a single doctor's visit, representing 125 times the EPA's reference dose for methylmercury. This calculation is particularly concerning given that some researchers have suggested that the reference dose should be set even lower for infants and fetuses.
Question 13: What is the relationship between timing of exposure and neurotoxic effects?
Research has demonstrated that developing brains are particularly susceptible to mercury damage, with the timing of exposure playing a crucial role in determining the severity and nature of neurological effects. Studies from the Faroe Islands showed that prenatal exposure to mercury resulted in cognitive deficits that persisted into adolescence, indicating that early exposure can cause permanent developmental changes.
The impact of timing is further emphasized by research showing that mercury exposure during critical developmental windows can disrupt fundamental processes like cell migration, differentiation, and synapse formation. This is particularly relevant to vaccine schedules, as the highest exposures often occurred during the most sensitive periods of brain development - the first six months of life - when the brain is undergoing rapid growth and organization.
Question 14: How does the body process and eliminate ethylmercury?
Studies have shown that while ethylmercury clears from the blood more quickly than methylmercury, this rapid clearance is actually due to its enhanced ability to penetrate organs and tissues, particularly the brain. Once in the brain, ethylmercury can be converted to inorganic mercury, which becomes effectively trapped in brain tissue with a half-life ranging from 227 to 540 days.
The Burbacher primate studies demonstrated that despite lower total mercury levels in the brain compared to methylmercury exposure, ethylmercury-exposed animals had higher levels of inorganic mercury, suggesting a more concerning pattern of accumulation and persistence. This challenges earlier assumptions that faster blood clearance of ethylmercury indicated greater safety compared to methylmercury.
Question 15: What role does glutathione play in mercury toxicity?
Glutathione serves as a crucial antioxidant and detoxification compound in the body's defense against mercury toxicity. Studies have found that individuals with glutathione deficiency may be particularly sensitive to Thimerosal exposure. A 2007 study by Geier and Geier found deficiencies in glutathione pathways in a group of children with autism who had received Thimerosal-preserved vaccines.
The relationship between glutathione and mercury toxicity is particularly significant because mercury exposure itself can deplete glutathione levels, potentially creating a dangerous cycle where initial exposure makes an individual more vulnerable to subsequent exposures. This interaction was not considered in many of the early safety studies of Thimerosal-containing vaccines.
Question 16: What are the documented cases of human harm from Thimerosal exposure?
Historical cases of Thimerosal toxicity include several documented fatalities. In the 1940s, when Thimerosal was used to treat patients with heart disease, at least one patient died from documented mercury poisoning. In 1969, five of six patients died after being injected with an antimicrobial containing abnormally high levels of Thimerosal. A 1983 report from the Ohio Board of Health strongly implicated Thimerosal in the death of a twenty-one-month-old child.
Beyond acute poisoning cases, numerous instances of neurological injury have been documented from therapeutic Thimerosal exposure. This includes a case of an eighteen-month-old girl who developed serious neurological injuries, including loss of coordination and unprovoked screaming behavior, following ear irrigation with Thimerosal-containing solution. Studies from the 1970s and 1980s consistently showed that 5-8% of patients using Thimerosal-containing products experienced adverse reactions.
Question 17: How do genetic factors influence individual susceptibility to mercury?
Research has indicated that genetic variations can significantly affect an individual's ability to detoxify and eliminate mercury from their system. Some individuals have genetic polymorphisms affecting glutathione production and function, making them more susceptible to mercury toxicity. This genetic variability helps explain why some individuals might be more severely affected by Thimerosal exposure than others.
Population-based epidemiological studies have been criticized for potentially missing these susceptible subgroups. The approach of studying entire populations, rather than focusing on potentially vulnerable subgroups, could mask significant adverse effects in genetically susceptible individuals. This limitation was highlighted in critiques of the European studies relied upon by the IOM in its 2004 report.
Question 18: What is the evidence for Thimerosal's role in autoimmune conditions?
Studies have shown that mercury exposure can trigger autoimmune responses in susceptible individuals. Research has linked mercury exposure to various autoimmune conditions, including rheumatoid arthritis and multiple sclerosis. The mechanism appears to involve mercury's ability to modify cellular proteins, potentially creating new antigens that the immune system then attacks.
Additionally, mercury has been shown to act as an endocrine disruptor and can interfere with immune system function at multiple levels. Clinical studies have documented cases of autoimmune reactions specifically triggered by Thimerosal exposure, with some research suggesting that even low-level exposure might contribute to the development of autoimmune disorders in susceptible individuals.
Question 19: What were the key findings of the original Verstraeten study?
The earliest analysis of the Verstraeten data in 1999 revealed striking correlations between Thimerosal exposure and neurodevelopmental disorders. Children receiving more than 25 micrograms of mercury in their first month of life were 11.35 times more likely to have autism, four times more likely to have ADD, and twice as likely to have developmental speech or language disorders compared to unexposed children.
The data showed a clear dose-response relationship, with higher levels of Thimerosal exposure correlating with increased risk of neurological problems. These initial findings were based on the review of medical records of more than 400,000 infants born between 1991 and 1997, making it one of the largest studies of its kind at the time.
Question 20: How did the Verstraeten study results change through multiple analyses?
The Verstraeten study underwent at least six major revisions between 1999 and 2003, with each subsequent analysis showing progressively weaker associations between Thimerosal and neurodevelopmental disorders. The relative risk for autism, for example, decreased from 7.62 in the first analysis to 1.69 in later versions, with various methodological changes contributing to this reduction.
These changes included excluding certain subgroups of children, altering inclusion criteria, and adding data from a third HMO with questionable record-keeping practices. The final published version in 2003 concluded there were no consistent significant associations, though Verstraeten himself later clarified that the results were "neutral" rather than negative, and that further research was warranted.
Question 21: What were the main criticisms of the European epidemiological studies?
The European studies suffered from fundamental exposure differences compared to the US vaccine schedule. Danish and Swedish children received substantially lower total mercury doses - around 75-125 micrograms compared to the US exposure of up to 237.5 micrograms. Additionally, European children typically started vaccinations at five weeks of age rather than at birth, meaning exposure occurred at less vulnerable developmental stages.
Another major criticism involved changes in autism diagnosis and reporting in Denmark. Before 1992, only inpatient cases were counted; after 1995, outpatient cases were included, artificially inflating autism rates after Thimerosal's removal. The Danish registry also excluded cases from one of Copenhagen's largest clinics prior to 1992, which accounted for approximately 20% of all Danish autism cases, further skewing the data.
Question 22: How did the Danish studies account for changes in autism diagnosis criteria?
The Danish studies failed to adequately account for significant changes in diagnostic criteria that occurred in 1994 when Denmark adopted a broader definition of autism. These changes, combined with the inclusion of outpatient cases after 1995, created the appearance of rising autism rates after Thimerosal's removal from vaccines in 1992.
The study authors claimed to have considered these administrative changes but did not publish their methodology for doing so. When researchers Trelka and Hooker corrected for these inaccuracies, they calculated that autism incidence had actually decreased significantly after 1993, including a dramatic 75% reduction within the 2- to 4-year-old cohort.
Question 23: What methodological flaws were identified in the key safety studies?
Major methodological flaws included the exclusion of approximately 25% of children from the Verstraeten study, many of whom represented potentially susceptible populations. The European studies suffered from incomparable exposure levels, different diagnostic criteria, and significant changes in autism reporting methods. The Andrews study used problematic statistical techniques that introduced multicollinearity issues between Thimerosal exposure and birth year variables.
The studies also failed to confirm their statistical power - the probability that they could detect real effects if present. This was particularly problematic in smaller population studies where insufficient sample sizes may have prevented the detection of significant associations. Additionally, many studies relied on population-level analyses that could have missed effects in genetically susceptible subgroups.
Question 24: How did the IOM evaluate the available research evidence?
The IOM's 2004 evaluation relied almost exclusively on epidemiological studies while dismissing pharmacological, clinical, and toxicological research. The committee focused narrowly on autism rather than considering the broader range of neurodevelopmental disorders potentially linked to Thimerosal. This represented a significant shift from their 2001 position, which had found the hypothesis "biologically plausible."
The committee refused to give weight to numerous peer-reviewed studies showing biological mechanisms of harm, instead relying primarily on five epidemiological studies with acknowledged methodological flaws. The IOM also declined to hear presentations from some researchers with relevant findings, including Richard Deth, who had just published evidence of Thimerosal's effects on gene regulation.
Question 25: What were the findings of the Geiers' VAERS analyses?
The Geiers' analyses of the Vaccine Adverse Event Reporting System (VAERS) found statistically significant increased risks for various neurodevelopmental disorders in children exposed to Thimerosal-preserved vaccines. Their 2003 study showed a two- to sixfold increase in neurodevelopmental disorders among children who received additional Thimerosal exposure compared to those receiving Thimerosal-free vaccines.
Further analyses in 2005 assessed Thimerosal exposure in approximately 110,000 children and found statistically significant associations between cumulative exposure and various disorders, including tics, ADD/ADHD, and speech and language delays. These findings were published in peer-reviewed journals and used accepted statistical practices, though they faced criticism from some in the scientific community.
Question 26: What evidence emerged from primate studies of Thimerosal?
Burbacher's pivotal 2005 primate study revealed that while ethylmercury cleared blood more quickly than methylmercury, it resulted in twice the levels of inorganic mercury in the brain. The study used doses comparable to the US vaccine schedule and demonstrated that the ethylmercury from Thimerosal was readily converted to inorganic mercury, which became trapped in brain tissue with a half-life of up to 540 days.
The primate studies also showed that Thimerosal-exposed infants had a higher ratio of brain-to-blood mercury levels than methylmercury-exposed infants. This research directly contradicted earlier assumptions about Thimerosal's safety based on blood clearance rates, suggesting that rapid blood clearance actually indicated faster transport to the brain rather than efficient elimination from the body.
Question 27: How did the CDC's access restrictions to the VSD affect independent research?
The CDC severely limited independent researchers' access to the Vaccine Safety Datalink (VSD), requiring congressional intervention for the Geiers to obtain even limited access. The CDC claimed that original data sets from the Verstraeten study had not been maintained and were essentially lost or destroyed, preventing independent verification of the study's evolving analyses.
These restrictions meant that the Geiers remained the only independent researchers who have ever queried the pre-2001 portion of the VSD regarding Thimerosal. Their findings, which showed consistent, significantly increased rate ratios for autism and other neurodevelopmental disorders, faced significant obstacles to publication and acceptance due to limited access to the complete database.
Question 28: What were the key findings from cellular and molecular studies?
Cellular studies demonstrated that Thimerosal could cause DNA breaks, membrane damage, and cell death in human neurons at concentrations comparable to those resulting from vaccine exposure. Research showed that Thimerosal was toxic to human cells at levels as low as 4.35 nanomolar concentration - below the conventional safety threshold of 29 nanomolar for mercury in umbilical cord blood.
Molecular studies revealed that Thimerosal disrupts critical cellular processes including methylation, DNA repair, and thioredoxin pathways. These disruptions affect fundamental developmental processes and cellular protection mechanisms. The research also showed that Thimerosal's toxicity was amplified in the presence of aluminum, another common vaccine ingredient, resulting in significantly higher rates of neuronal death when the two substances were combined.
Question 29: Why did the FDA ban Thimerosal in over-the-counter products but not vaccines?
The FDA banned Thimerosal in over-the-counter products in 1998 after determining it was not "generally recognized as safe and effective." This decision followed years of research showing Thimerosal's potential for cell damage and allergic reactions. However, the FDA maintained different standards for vaccines, creating an apparent contradiction where a substance too dangerous for topical use was still permitted for injection into infants.
This discrepancy may be explained by poor communication between different FDA divisions. According to Dr. Sarah Bridges, an FDA scientist revealed that there was little communication between the group responsible for over-the-counter medications and the group regulating vaccines. This organizational disconnect may have contributed to the inconsistent policy regarding Thimerosal's use.
Question 30: How did different countries respond to Thimerosal safety concerns?
Several countries took decisive action to remove Thimerosal from vaccines well before the United States. Denmark eliminated Thimerosal in 1992, Sweden in 1989, and the United Kingdom completed its phase-out by 2004. Other countries including Australia, Canada, and France announced intended phase-outs and expressed preferences for Thimerosal-free formulations for children.
Notably, these countries maintained their decisions to keep Thimerosal out of vaccines even after studies conducted in their populations allegedly showed no harm from the preservative. This suggests that these nations were applying the precautionary principle, choosing to err on the side of safety despite the higher costs associated with alternative preservation methods.
Question 31: What was the significance of the Simpsonwood meeting?
The Simpsonwood meeting, held in June 2000 near Atlanta, gathered fifty-three individuals including high-ranking CDC and FDA representatives, state public health officials, and vaccine manufacturer representatives. The meeting was conducted without public notice to discuss Verstraeten's findings showing significant correlations between Thimerosal exposure and neurodevelopmental disorders. Verstraeten introduced his research by acknowledging statistically significant relationships between Thimerosal and neurological disorders.
At this meeting, Verstraeten noted that his data likely understated the connection between Thimerosal and conditions like speech delays and ADD because many children in the study were too young to be diagnosed. Following this meeting, subsequent analyses of the data showed progressively weaker associations between Thimerosal and neurodevelopmental disorders, raising questions about potential data manipulation.
Question 32: Why do flu vaccines still contain Thimerosal?
Flu vaccines continue to use Thimerosal primarily because they are often manufactured in multidose vials, which manufacturers claim are more cost-effective. According to CDC data from the 2013-2014 flu season, 39% of available flu vaccine doses contained Thimerosal. Health maintenance organizations and state health departments generally opt for multidose vaccines because they are less expensive than single-dose shots.
This continued use persists despite the fact that Thimerosal-preserved vaccines must be disposed of as hazardous waste due to their mercury content. The mercury levels in these vaccines exceed regulatory levels for hazardous waste classification, creating an apparent contradiction where a substance that must be handled as toxic waste can still be injected into pregnant women and children.
Question 33: How have state-level policies on Thimerosal evolved?
Several states have passed laws aimed at reducing Thimerosal exposure to pregnant women and young children. California, Delaware, Illinois, Iowa, Missouri, New York, and Washington have enacted legislation requiring vaccines to contain no Thimerosal or only trace amounts when administered to infants, children, and pregnant women. California's law, effective July 2006, specifically banned Thimerosal-preserved vaccines for children under three and pregnant women, except for flu vaccines.
Other states including Maryland, Massachusetts, and Rhode Island have attempted similar bans. These state-level actions reflect growing concern about mercury exposure through vaccines, despite federal agencies' continued assertions of Thimerosal's safety. The variations in state policies have created a patchwork of regulations regarding Thimerosal use across the United States.
Question 34: What was the CDC's response to manufacturers' offers to produce Thimerosal-free vaccines?
Shortly after the July 1999 AAP/USPHS statement calling for Thimerosal's removal, two major vaccine manufacturers wrote to the CDC indicating they could supply sufficient Thimerosal-free versions of their products to meet total vaccine demand. Merck offered to accelerate production of its Thimerosal-free hepatitis B vaccine, while SmithKline Beecham (now GlaxoSmithKline) offered to meet US market needs for Thimerosal-free DTaP.
The CDC, however, rejected these offers. CDC director Jeffrey Koplan responded that they would "continue to provide the States with a choice among currently licensed brands of DTaP vaccine." This decision effectively delayed the transition to mercury-free vaccines, allowing existing Thimerosal-containing stocks to remain in use until 2003.
Question 35: How did the IOM's position on Thimerosal change between 2001 and 2004?
In 2001, the IOM concluded that a link between Thimerosal and neurodevelopmental disorders was "biologically plausible" and recommended that "full consideration be given to removing Thimerosal from any biological or pharmaceutical product to which infants, children, and pregnant women are exposed." This position acknowledged the potential risks and advocated for a precautionary approach.
By 2004, the IOM's position shifted dramatically, favoring "rejection of a causal relationship between Thimerosal-containing vaccines and autism." The committee recommended against further research into vaccine-autism links and suggested available funding be directed elsewhere. This reversal was based primarily on epidemiological studies, while largely dismissing the growing body of biological and toxicological evidence.
Question 36: What role did conflicts of interest play in policy decisions?
Significant conflicts of interest existed among researchers involved in key studies. For example, Thomas Verstraeten, lead author of the influential CDC study, took a job with GlaxoSmithKline, a vaccine manufacturer, while the study was still ongoing. The Danish studies were conducted by researchers with direct ties to vaccine manufacturers or receiving funding from national vaccine agencies, including the CDC.
Dr. Poul Thorsen, a co-author of the Madsen study, developed a long-term relationship with the CDC and received substantial research funding. He was later indicted for wire fraud and money laundering of more than $1 million from the CDC. Elizabeth Miller, involved in UK studies considered by the IOM, had received funding from various vaccine manufacturers but did not specifically disclose these relationships when presenting her results.
Question 37: What alternatives to Thimerosal exist as vaccine preservatives?
2-phenoxyethanol (2-PE) has emerged as a primary alternative to Thimerosal and is already being used in several vaccines including the polio vaccine Ipol. A 2010 study demonstrated that 2-PE is approximately seventy times less toxic than Thimerosal to human cells compared to bacterial cells when tested at vaccine-preservative levels. Research from Pfizer found that "Thimerosal is not an effective preservative compared to 2-PE."
Beyond alternative preservatives, single-dose vials eliminate the need for preservatives entirely. While manufacturing single-dose vials involves higher initial costs, studies suggest this approach might be more cost-effective when considering factors such as reduced waste and improved vaccination compliance. Some countries have successfully transitioned to preservative-free, single-dose formulations for their entire vaccine programs.
Question 38: How does 2-phenoxyethanol compare to Thimerosal in safety and effectiveness?
Research has shown that 2-PE is substantially safer than Thimerosal while providing better antimicrobial protection. A 2010 study demonstrated that 2-PE is about seventy times less toxic to human cells than Thimerosal when compared at preservative levels. Additionally, joint research by Pfizer found that 2-PE was more effective as a preservative than Thimerosal.
2-PE is already successfully used in several vaccines, including DTaP, DTaP-IPV/Hib, and Tdap vaccines at non-preservative levels, and in the polio vaccine Ipol as a preservative. These real-world applications demonstrate both the feasibility and safety of transitioning away from Thimerosal to alternative preservatives.
Question 39: What are the practical implications of switching to single-dose vials?
The transition to single-dose vials would eliminate the need for preservatives entirely but requires changes in manufacturing, storage, and distribution systems. While single-dose vials initially cost more to produce and require more cold-storage space, studies have shown that these costs may be offset by reduced vaccine wastage, which can be as high as 60% with multidose vials.
Healthcare workers have shown reluctance to open multidose vials for vaccinating only one or two children, leading to missed vaccination opportunities. Single-dose vials eliminate this concern while also reducing contamination risk. The PATH study in 2003 suggested that single-dose vials might actually be more cost-effective when accounting for wastage and improved vaccination coverage.
Question 40: How have some countries successfully eliminated Thimerosal from their vaccine supplies?
Denmark, Sweden, and the United Kingdom have successfully eliminated Thimerosal from their vaccine programs through systematic approaches prioritizing public health over short-term economic considerations. Denmark removed Thimerosal from vaccines in 1992, Sweden in 1989, and the UK completed its phase-out by 2004. These countries maintained their Thimerosal-free policies even after studies conducted in their populations allegedly showed no harm.
These countries demonstrated that transitioning to Thimerosal-free vaccines is practically achievable and sustainable. Their success provides a model for other nations, showing that concerns about cost and logistics can be overcome with proper planning and commitment to public health priorities. Notably, these countries have maintained high vaccination rates without compromising vaccine effectiveness or safety.
Question 41: What are the cost differences between single-dose and multi-dose vaccine production?
Initial manufacturing costs for single-dose vaccines are higher than multidose versions, with production costs around $0.25 per single-dose vial compared to $0.10 for a ten-dose vial. The cost difference stems from increased packaging materials, additional cold storage requirements, and more complex manufacturing processes. Advocates of multidose vials frequently cite these cost differentials as justification for continuing Thimerosal use.
However, these basic cost comparisons fail to account for significant hidden expenses associated with multidose vials. When factoring in the documented 60% wastage rate of multidose vials, along with additional cold chain requirements and waste disposal costs for mercury-containing products, the actual cost differential becomes much less significant. Healthcare workers' reluctance to open multidose vials for small numbers of patients further impacts the real-world cost efficiency of this format.
Question 42: How does vaccine wastage affect the economics of different packaging options?
Studies have shown that wastage rates for multidose vials can reach 60%, significantly undermining their apparent cost advantages. This high wastage rate occurs because opened vials must be discarded after a certain period, even if doses remain unused. Healthcare workers' hesitancy to open new multidose vials for small numbers of patients also contributes to missed vaccination opportunities and effective wastage.
The PATH study in 2003 concluded that when accounting for all factors, including wastage, single-dose vials may actually be more economical, particularly for expensive vaccines. The study also found that single-dose formats led to better vaccination coverage as healthcare workers were more willing to vaccinate small numbers of children, effectively improving the overall cost-effectiveness of vaccination programs.
Question 43: What are the estimated costs of removing Thimerosal globally?
The World Health Organization estimates that switching to single-dose from multidose vials would raise annual costs to UNICEF or PAHO by approximately $300 million. This figure includes increased costs in manufacturing, shipping, cold-chain storage, administration, and waste-handling infrastructure. These estimates have been used to argue against global Thimerosal removal.
However, these costs must be weighed against the potential societal costs of mercury exposure. In the United States alone, the annual economic costs of autism were estimated at $137 billion as of 2012, according to Autism Speaks. When considering the potential global health impact and associated economic burden of mercury exposure, the $300 million cost of Thimerosal removal appears relatively modest.
Question 44: What are the economic implications of potential mercury-related health effects?
A 2005 study by Trasande calculated that mercury-related IQ losses in affected populations could result in diminished lifetime economic productivity amounting to $8.7 billion per birth year cohort. This calculation was based on documented IQ losses ranging from three-quarters to more than three points in children with elevated mercury exposure levels.
The annual cost of autism in the United States alone was estimated at $137 billion in 2012, encompassing factors such as lost family income and productivity as well as direct care costs. While not all these costs can be attributed to Thimerosal exposure, even a small reduction in neurodevelopmental disorders resulting from eliminating this known neurotoxin could produce significant economic benefits that far outweigh the costs of transition to alternative preservation methods.
Question 45: How do cold chain requirements affect preservative choices?
Cold chain requirements significantly impact vaccine costs and logistics, with both single-dose and multidose formats requiring careful temperature control during storage and transport. While multidose vials initially require less cold storage space per dose, their higher wastage rates mean that more vials must be stored and transported to achieve the same effective vaccination coverage.
The World Health Organization has noted that cold chain considerations contribute to the estimated $300 million annual cost increase for switching to single-dose formats globally. However, improved cold chain management systems and more efficient storage solutions continue to be developed, potentially reducing these costs over time. Additionally, the reduced wastage associated with single-dose vials may help offset some cold chain expenses.
Question 46: Why did the CDC restrict access to the Vaccine Safety Datalink?
The CDC severely limited independent researchers' access to the Vaccine Safety Datalink (VSD), requiring congressional intervention for researchers like the Geiers to obtain even limited access. The agency claimed that original data sets from the Verstraeten study had not been maintained and were essentially lost or destroyed, preventing independent verification of the study's evolving analyses.
This restriction meant that only CDC-affiliated researchers could fully access and analyze the database, raising concerns about potential bias in vaccine safety research. The Geiers remained the only independent researchers who have ever queried the pre-2001 portion of the VSD regarding Thimerosal, despite the database being funded by public money and presumably meant to serve public health interests.
Question 47: How did changing diagnostic criteria affect autism prevalence studies?
Changes in diagnostic criteria significantly impacted autism prevalence data, particularly in the Danish studies. In 1994, Denmark adopted a broader definition of autism, while simultaneously switching from counting only inpatient cases to including outpatient cases in 1995. These changes artificially inflated autism rates after Thimerosal's removal from vaccines in 1992, potentially masking any protective effect from removing the preservative.
When researchers Trelka and Hooker corrected for these diagnostic and reporting changes, they calculated that autism incidence had actually decreased significantly after 1993, including a dramatic 75% reduction within the 2- to 4-year-old cohort. This analysis suggests that changes in diagnostic criteria and reporting methods may have obscured the relationship between Thimerosal exposure and autism rates.
Question 48: What role did publication bias play in the scientific discourse?
Publication bias significantly influenced the scientific discourse surrounding Thimerosal safety. The CDC-sponsored Verstraeten study, for example, underwent multiple revisions before publication, with each version showing progressively weaker associations between Thimerosal and neurodevelopmental disorders. Additionally, some researchers with relevant findings, such as Richard Deth, were denied the opportunity to present their research to the IOM.
The Price study, which was eventually published in Pediatrics, was initially rejected by both JAMA and the New England Journal of Medicine, suggesting concerns about its methodology or conclusions. These publication patterns raise questions about whether the published literature accurately reflected the full scope of scientific evidence regarding Thimerosal safety.
Question 49: How did different statistical methodologies affect study outcomes?
Statistical methodology choices significantly impacted study outcomes. The Andrews study used a problematic multivariate regression technique that introduced multicollinearity between Thimerosal exposure and birth year variables, potentially masking true associations. The Verstraeten study's multiple revisions included changes in statistical analysis methods that progressively reduced the apparent strength of associations between Thimerosal and neurodevelopmental disorders.
Studies also varied in their treatment of potential confounding factors and in their choices of statistical significance thresholds. The European studies were criticized for lacking sufficient statistical power to detect real effects, particularly in smaller population subgroups. These methodological variations make it difficult to draw firm conclusions from the epidemiological literature.
Question 50: What factors influenced the interpretation of population-level studies?
Population-level studies were influenced by numerous factors that complicated their interpretation. These included variations in exposure levels between countries, differences in vaccination schedules, changes in diagnostic criteria, and modifications in reporting methods. The Danish studies, for example, were conducted in populations receiving substantially lower mercury exposures than US children and used different diagnostic and reporting criteria over time.
Additionally, population-level analyses may have missed effects in genetically susceptible subgroups by averaging outcomes across entire populations. The exclusion of potentially susceptible populations, such as the 25% of children excluded from the Verstraeten study, further complicated the interpretation of these studies. These limitations suggest that population-level studies alone may be insufficient to establish Thimerosal safety.
I appreciate you being here.
If you've found the content interesting, useful and maybe even helpful, please consider supporting it through a small paid subscription. While everything here is free, your paid subscription is important as it helps in covering some of the operational costs and supports the continuation of this independent research and journalism work. It also helps keep it free for those that cannot afford to pay.
Please make full use of the Free Libraries.
Unbekoming Interview Library: Great interviews across a spectrum of important topics.
Unbekoming Book Summary Library: Concise summaries of important books.
Stories
I'm always in search of good stories, people with valuable expertise and helpful books. Please don't hesitate to get in touch at unbekoming@outlook.com
For COVID vaccine injury
Consider the FLCCC Post-Vaccine Treatment as a resource.
Baseline Human Health
Watch and share this profound 21-minute video to understand and appreciate what health looks like without vaccination.









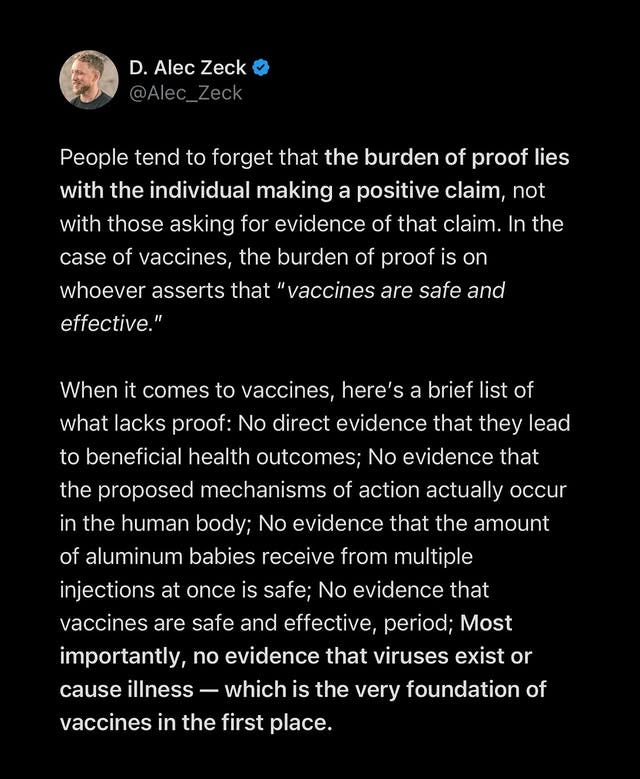



"This is straight after Bobby had just told me that I had injected mercury into my kids...
When you’re brainwashed, you’re brainwashed."
☝️This was me also, despite studying biology in uni...
I was brainwashed about vaccines, I never questioned it, because they were a minor part of life, you get one or don't, big deal... I really didn't give it another thought, after all they'd been researched thoroughly right?
It took the "we're going to inject man made mRNA into everyone for an unexplained cold" schtick for me to suddenly stop and say, "Hang on... WTF??"... At least I remembered what I learnt in microbiology. mRNA transcription and translation is fundamental to the health, and function of your cell.
Anything that interferes with the day to day operations of your cells, anything that interferes with the unique and delicately timed genetic expression of your cell is deadly... is deadly by design... There is no way that we know enough to interfere with that safely.
Apart from the fact it is immediate autoimmunity, straight off the bat, from there it just gets worse.
I started to wake up, then so many other things made no sense, the hype...
The "take it or else"
Jonestown vibes right there.
I was freaking out for the health of anyone who took this, why would the health authorities I trusted implicitly be pushing this..?.
Why were they saying "safe and effective" when that couldn't possibly be known, nor proven?
Why was everyone on message around the world?
Why were vaccines suddenly the most important thing ever, they were always such a minor part of life?
Why no questions allowed?
Why no choice?
It's a cold, if it really was even that (another story there) so why the DESPERATION for you to take one?
There was no way this could work without primarily destroying cellular operations critical for day to day health.
All scientists should KNOW this... They DO KNOW this... Why would anyone ALLOW this?
It beyond reckless, it's murder, because this method of creating antibodies was toxic, the antibodies were secondary as a response to an attempt to kill cellular function.
Yet again they KNOW this... This is literally the most dangerous way to create useless antibodies I can think of, destroy the function of, even kill the cells of a multicellular organism to fight a cold that would have been harmless by the time the vaccines were shipped, it made zero sense.
Given they've found the mRNA is hardy enough to produce spike protein well over two years later from a double dose, one suspects anyone who got a good dose will have a shortened lifespan, many have already succumbed. Plausible deniability for the criminals of course as a lot will take years to manifest.
Plus it created IgG antibodies instead of IgA antibodies required for a respiratory pathogen in the first place (so it could never work - even though I'm mostly convinced covid itself was a sham).
Okay at this point I'm awake (a bit) and find the world I thought I was living in was a sham, either that or I'd fallen into a twilight zone of upside down reality.
Since then I thought about ALL the vaxs I'd had... holy cow... Even the bullshit about aluminium as an immune system "stimulant"... What a crock... As you pointed out... Bloody hell...
Crazy that my uni biology hadn't made me think about it until the great mRNA push (let alone the plasmid DNA discovery, and endotoxin in all jabs, but especially the "covid" ones)... Kicking myself for not looking closely before.
Thank you for your hard work in pumping these articles out for us to join the dots about the real world. I save almost every article you write to keep as a reference.
"Vaccinations are among the most important advances in medicine in the last century. We have eradicated smallpox from the planet and dramatically reduced death and suffering from infectious disease around the globe. I am aggressively pro-vaccine, as are the editor and introduction contributor of this book. I am a father and family physician. I have vaccinated my children. I have been vaccinated and recommend vaccination to my patients." Mark Hyman had not gotten the message in 2015 when he wrote this, but who had? Here is a draft post, 345. IT IS HARD TO GROK* HOW EVIL VACCINES ARE https://robertyoho.substack.com/publish/post/154000018