Inside Big Pharma: Interview with Hedley Rees
On Supply Chains, Depopulation, Gene Therapy, Generic Medicines, Penicillin Development, Patent Reform and much more.
Industry insiders that tell the truth are exceedingly rare.
Hedley Rees brings over 40 years of pharmaceutical industry experience as a former global head of supply chain at Bayer and consultant to numerous biotech companies.
In our interview, he shares critical insights about the transformation of drug manufacturing, the weakening of regulatory oversight, and the technical realities of pharmaceutical supply chains. His unique expertise offers an invaluable perspective on how the industry has evolved to today's profit-driven model.
With thanks to Hedley Rees.
1. Hedley, can you please share your background and journey, and what sparked your interest in challenging the conventional narratives of the pharmaceutical industry?
Well, it’s a long story, but I’ll try to keep it as short as possible. It can be divided into five phases of my career:
PHASE 1: Life in Pharmaceutical and Biopharmaceutical Manufacturing and Distribution
1975 - Degree in production engineering from University of Wales, four years working in automotive, consumer durable, and heavy engineering (steel) sectors.
1979 – Joined Miles Laboratories in Wales, which was taken over by Bayer AG in 1980. The site was a state-of-the-art manufacturing plant making pharmaceutical and diagnostic products distributed globally. That included sterile injectable products, the dosage form used for the SARS-CoV-2 injections.
I joined as an industrial engineer (improvement of industrial systems) and worked my way up over 16 years to global head of supply chain. Bayer sponsored me through an executive MBA at Cranfield in 1994/5, after which I was recruited by British Biotech, in the role of head of logistics.
PHASE 2: Life in Biotech
First, the term ‘biotech’ needs clarification, as there is a significant amount of confusion as to the meaning, even among those in the industry. The original term ‘biotech’ was applied to the business model that was formed once Big Pharma outsourced its physical assets required to develop new drugs. The drug development contract organisations that were formed in response to the outsourcing could then be used by small drug development companies. Their aim would then be to sell a part developed drug to Big Pharma, making a profitable ‘exit’. The drugs those small companies were developing were the simpler small molecule products, manufactured using chemical synthesis.
British Biotech (see below) was originally called British Biotechnology when it was first formed in the 1980s, because the technology was beginning to emerge. However, the only drug assets available at the time were based on small molecule processing. British Biotechnology therefore shortened its name to British Biotech.
Being a biotech meant that to develop a drug, they had to raise a lot of money to pay the contractors. British Biotech raised c. £400 million, which was an impressive sum of money at the time. More can be found out on the company here.
I left to join a second biotech company when British Biotech went bust. The Board had conned financial analysts and investors into believing they were developing drugs with blockbuster potential. They lied about everything, and proved, to me at least, how easy it was to con people about drugs.
After heading up the supply chain activities for two further biotech companies, both of which brought a new drug to market for migraine and non-small cell lung cancer, I left to become a supply chain consultant in 2005, setting up PharmaFlow, which I still run.
PHASE 3: Life as a Supply Chain Consultant
Life as a supply chain consultant in biotech was an education and a half! By this time (2005), Big Pharma had completely retreated into discovery research and sales & marketing, waiting for biotech companies to develop the drugs on their behalf. The problem was that the biotech’s were full of scientists wanting to be millionaires, even billionaires, but had no idea about how supply chains should be built and managed. They also could never have raised enough cash to pay for all the work required.
Those that asked me to help them soon went away when I explained the problems they had created for themselves. They went elsewhere to find consultants that would tell them what they wanted to hear. I didn’t make a whole lot on money, as you might have expected.
PHASE 4: Life as a Campaigning Author
In 2008, I was approached by Wiley to write a book on the pharmaceutical supply chain, which published in 2011.
That book was 450 pages, going into great depth on how to develop, manufacture and distribute drugs. It sold in 35 countries, US being the highest sales, but not in huge quantities anywhere in the world. It did, however, get me the reputation inside the industry as being a thought leader for radical reform, to the extent where UK Government’s Office for Life Sciences asked me to consult to a gene therapy development and manufacturing organisation in Oxford. The project was a funding call known as the Advanced Manufacturing Supply Chain Initiative (AMSCI). They got £7.1 million funding from it, which was pretty much down to the work I had done for them.
The UK Government was very interested in the potential of gene therapy, even though I was clear that the supply chain had massive challenges to be surmounted. It seems they didn’t listen.
From then on, I decided to self-publish easy-to-read accounts of all the issues, below:
2015: Find It, File It, Flog It: Pharma's Crippling Addiction and How to Cure it
2019: Taming The Big Pharma Monster: by Speaking Truth to Power
2022: THE COVID-19 SUPPLY CHAIN: Fact not Fiction
They didn’t sell more than a handful of copies; not unexpected.
PHASE 5: Life during the SARS-CoV-2 Injections Scam
The answers to the questions that follow will paint the picture of the last four years under the COVID regime.
2. In your research, you discuss potential depopulation agendas and profit motives. How did you arrive at these conclusions, and what evidence supports them?
I should first say something about my research methodology, which has been described by a reviewer of the manuscript for my next book for Wiley, Transforming the Pharmaceutical Supply Chain, as Action Research. If anyone wants to look it up, search for Kurt Lewin: https://en.wikipedia.org/wiki/Kurt_Lewin
In simple terms, it is learning by doing. PHASES 1 – 4 of my career allowed me to learn what was going wrong in the pharmaceutical industry, and how to put it right. I’ve been publishing on that, through Wiley and self-publishing on Amazon KDP, for the last 15 years.
This current PHASE 5 of my career has provided an opportunity to spread that learning to a much wider audience. To date, however, it has not fully cut through to the right quarters. My theory is that the myth of penicillin, perpetuated by pharmaceutical companies, has led the world believing drugs come to market ‘by accident’. You will learn why that is not the case as you read on.
The following clarification on developing new drugs is taken from the draft manuscript submitted to Wiley:
In the chapters that follow, I shall prove that creating a medicine requires the same blend of skills, knowledge and experience as developing an aircraft, automobile, aero-engine, or silicon chip. In proving that, this book’s ambition is to convince readers that there is not a moment to lose. The wheels of change must begin to turn with ever greater velocity, as healthcare professionals, and the patients they serve, are empowered to regain their rightful place in the development of new drugs.
I live in hope that PHASE 6 of my career, if I am spared a little longer, will consist of spreading the messages far and wide, so that the greatest crime against humanity in the history of the world, will never, ever, be repeated.
Now, onto the main body of the question. If we accept that C19 was as I have just described it, then it deserved investigation akin to a scene-of -the -crime investigator. Motive may be important in catching the crooks down the line, but initially it is the facts and evidence collected where the crime took place that must take precedence. The reason being that if detectives collared criminals based on motive alone, there would be a ton of innocent people locked up in jail…
…and the guilty walking around free.
So, where was the crime scene? It was the vaccination centres and various other temporary constructions set up around the world to inject innocent, unsuspecting people. That’s where the injections did the horrible catalogue of damage.
A visit to the scene by experts in the manufacturing and distribution supply chain would have unearthed a host of manufacturing activities being carried out by unqualified staff, with no standard operating procedures to ensure they comply with safe working practices. Without the necessary skills and proper manufacturing instructions, it would be hit-and-miss if the drug administered was safe, or not. That is aside from the toxic nature of some of the materials, such as adjuvants, used to ‘help’ the injections work better. On-site analysis of a sample of doses administered to patients, by REAL experts, would have stopped the whole thing in its tracks.
Then, supply chain experts would trace upstream in the supply chain, to the manufacturing facilities that were producing the injections and shipping them into to vaccination centres. In my Substack INSIDE PHARMA, there is a body of evidence where FDA has inspected those sites and found them to be severely deficient against pharmaceutical standards. This provides some detail:
Read this: Moderna's new booster launch tripped up by production issues at Catalent plant: reports
And this, for more details:
https://www.fda.gov/media/161643/download
It is therefore my contention, based on the above and further evidence in INSIDE PHARMA, that this was not a depopulation agenda. Neither were the military involved to any extent, nor were the injections designed to kill, as bioweapons, or any of the other, accidental or purposeful distortions.
Many involved, such as Sir Lord Patrick Vallance, Sir Chris Whitty and Sir Pascal Soriot (CEO AstraZeneca) were rewarded handsomely with honours and pay awards. They were certainly not doing what they did to reduce the world’s population.
As a rough gauge, we might ask ourselves, after four years of ‘depopulation’ how many people are still left of the planet? Is it still around 8 billion? Or no?
3. You’ve been critical of mRNA technology’s rapid adoption. What specific aspects of its development and distribution do you find most concerning?
This is a really good question – one that requires some background information on a project I consulted on in 2013. This is documented in INSIDE PHARMA, so I can use the excerpts that follow to explain.
In early 2013, I was contacted by the Technical Director of the UKs HealthTech and Medicines KTN (as it was then), now Health KTN. He explained that no UK Life Sciences company had been successful in bid submissions to the first two rounds of the UKs Advanced Manufacturing Supply Chain Initiative (AMSCI). He went on to say that the Office for Life Sciences was not happy with it. The feedback they had received was that the bids were heavy on science but showed little understanding of the manufacturing supply chain. That was not a surprise to me!
Given my background, he asked if I would be open to a 4-day consultancy project to find a UK based life sciences company that could fit the bill as a candidate to submit a bid for Round 3. The brief was to attend the launch meeting for Round 3 and 4, then use my extensive network in biopharmaceutical supply chain strategy and management to identify a target company and sign them up.
The launch meeting was held at The QEII Centre, the largest dedicated conference and exhibition space in central London. The Government account of this £120 million funding competition for manufacturing supply chain companies stated:
“AMSCI is a funding competition designed to improve the global competitiveness of UK advanced manufacturing supply chains. £120 million is available for rounds 3 and 4, and the competition is open to all organisations that are part of a manufacturing supply chain. This funding is available to support research and development, skills training and capital investment. It will help UK supply chains achieve world-class standards and encourage major new suppliers to locate in the UK.”
On the day of the meeting, we were sat in tables of 8 people around the room. Michael Fallon, one of a breed of politicians we no longer see (IMHO only), gave an excellent opening address. As the day progressed, people around the table began to chat, as they do. On our table, a gentleman representing a low carbon vehicle developer, began to talk about the biotech industry. Curious as ever, I asked him how he knew about it.
“I’m the Chairman of Oxford BioMedica”, he revealed…
…and the day ended with me being invited to visit the site to meet with Oxford BioMedica’s Director of Manufacturing.
It wasn’t difficult to find the site in Cowley. I had previously worked as a senior manager at the site for two previous owners, British Biotech and OSI Pharmaceuticals. It overlooked the BMW manufacturing plant in Cowley, where we could watch the Mini’s rolling out, in various colours and shades, by the hundreds and thousands.
Oxford BioMedica (OXB) had purchased a biologics manufacturing facility on the site, known as Harrow House.
OXB was then, and still is, a Contract Development and Manufacturing Organisation (CDMO, providing scale-up solutions and commercial supply of viral vectors to pharmaceutical and biotech companies in the fast-growing cell and gene therapy field.
The day went well, as the Manufacturing Director was up for new ways of working, and surprise, surprise, the Head of Supply Chain had been a master production scheduler in my team at Bayer. I left with commitment from them to the drafting of a consultancy agreement for signature. Within a week I was signed up.
Work begins with ‘what is’
As ever, I begin the assignment with a current state assessment. This involves mapping the inbound supply chain, the internal manufacturing flows, and the outbound journey to the next stage. The various items to be procured are identified, their suppliers, and data such as lead times and shipping conditions (mainly cold chain) are collected and documented.
Inside the plant, data on the stages in the process flow, batch sizes, production cycle times, waiting times, and a significant amount of other data were collected and documented, and so it went for outbound.
Now it’s the ‘to be’
Now we had detailed the current state supply chain, it was time to work out the future state, or what is ‘to be’.
For this bid to be classed successfully as ‘an advanced manufacturing supply chain initiative’, it had to be something of exceptional merit, given no other life sciences company had achieved a successful bid to date. I was confident though, because the company had good skilled people and the Director of Manufacturing was fully on board.
Knowing this was a gigantic challenge, I reached into my LinkedIn network for a suitable ally. It turned out to be a Royal Academy of Engineering Professor at Cranfield University—a former medic who had turned to designing Jaguar Cars! Perfect for an academic partnership on the project. He is still a very good friend today.
He put me in touch with a fellow Welsh person, who happened to be Head of Innovation at a hospital network in the West Midlands, UK. Another perfect partner to provide the NHS perspective. She is still a very good friend today.
Together, we were ready to work with OXB to deliver an end-user focussed model for developing gene therapy products—and the Office for Life Sciences (part of the Department for Business, Innovation and Skills (BIS) at the time) was delighted with the prospect!
How did it turn out?
Cutting to the chase, the bid was successful and OXB received the funding sought, see the Press Release below:
Oxford BioMedica Wins Significant Funding via a Competitive Award from UK Government’s Advanced Manufacturing Supply Chain Initiative. Here is an extract:
Oxford, UK – 11 September 2013: Oxford BioMedica plc (“Oxford BioMedica” or “the Company”) (LSE: OXB), the leading gene-based biopharmaceutical company, announces that it has been selected as a winner of a funding award under the UK Government’s Advanced Manufacturing Supply Chain Initiative (AMSCI), in recognition of the Company’s potential to become a world-leader in Advanced Therapy Medicinal Product (ATMP) manufacture and supply chain expertise.
Oxford BioMedica led the successful bid with four other UK-based participants: the Heart of England NHS Foundation, Cranfield University, Cell Therapy Catapult Ltd and Biotec Services International Ltd (together, the “consortium”). Subject to due diligence and final confirmation by Birmingham City Council, the consortium has been awarded a £2.4 million grant, of which Oxford BioMedica will receive £1.8 million, and a £5.3 million loan to Oxford BioMedica which is repayable by March 2017.
***
Fast forward to 2020, my jaw dropped to the floor I realised Oxford BioMedica was developing the AstraZeneca drug substance, using the same viral vector technology that I had worked on in 2013. Then, there were massive hurdles to be surmounted before the supply chain for such an experimental technology could be regarded as anywhere near safe. When I started looking back at the Press Releases, I realised that MHRA had approved OXB’s manufacturing facility at breakneck speed, without ever physically visiting the site.
As I write this today, my research tells me that viral vector technology is gene modified cell therapy, as is the mRNA technology. It is genetic engineering via the side entrance. It also tells me that the Novartis gene therapy for rare blood cancers, which I worked on in 2013, and was FDA approved in 2017, was rebranded by AstraZeneca as a ‘VACCINE’. It was the same for BioNTech/Pfizer and Moderna – gene modified cell therapy. Confirmatory evidence that the motive was to earn blockbuster revenues, not depopulate the earth.
That is a long answer to a short question, but if you don’t know the story, why would you believe me?
4. Serialization is a standard in the pharmaceutical supply chain, yet it seems it wasn’t followed for mRNA vaccines. Why do you think this deviation occurred, and what are the implications?
The vials of frozen injections could never have been serialized because there were multiple doses inside the vial. Never before had a nurse of doctor received anything other than a single vial with a single dose inside to administer to a patient. Storage temperature would not be lower than refrigerated. With the frozen SARS-CoV-2 injections, further manufacturing operations were required to get to the final dose administered to the patient. The principle behind serialization is that the final dose of drug produced by the manufacturer can be tracked to the patient receiving it, but the manufacturer did not carry out the finishing operations.
There is also a broader issue with serialization in general. It only applies to finished products and the Government Acts passed, such as the EUs Falsified Medicines Directive of 2011, and US Drug Supply Chain Security Act (DSCSA) are still not fully observed. In other worlds, serialization as it has been implemented, is not working effectively, or safely.
You might ask yourself, why does it take a Government Act to tell big pharma companies how to manage their supply chains?
5. You advocate for empowering doctors to repurpose generic medicines. How realistic is this approach, and what barriers do they face in implementing it?
It is extremely realistic, see:
What’s stopping doctors repurposing generic medicines? Answer – not a lot!
“For decades, it has been assumed only pharmaceutical companies have the wherewithal to develop or repurpose medicines. This article challenges that assumption based on changing business models in the industry. These changes have created a skills-base of qualified contractors and contract organisations able to offer everything required to bring a medicine to market.
The message to doctors, other health professionals and healthcare systems (referred to as doctors from here on) is that there is just one gap in their armoury—knowledge of the supply-chain that delivers medicines to patients.”
Over the past four decades, physicians have been elbowed out of the development of drugs. As an example, Alexander Fleming, who identified the potential of penicillin, was a physician. However, he needed Oxford University to isolate the active substance, and Andrew Moyer of the USDA to define the manufacturing process – it took 16 years in total. See:
6. Based on your experience, how has the pharmaceutical industry’s focus shifted from patient outcomes to profit motives over the years?
This is the opening of the final chapter, Chapter 22, of my next book to be published by Wiley, NJ, in the New Year, titled Transforming the Pharmaceutical Supply Chain:
Returning to Purpose
“We try never to forget that medicine is for patients. We try never to forget that medicine is for the people. It is not for the profits. The profits follow, and if we have remembered that, they have never failed to appear. The better we have remembered it, the larger they have been!”
George W. Merck, President and Chairman Merck & Co., Inc. (1925-1957).
These words from George W. Merck were delivered in 1951, 30 years before the blockbuster era began. Merck’s reasoning appears to be sound, as any business that puts its consumers first is likely to enjoy a loyal customer base, repeat business and a fair return on investment.”
In summary, the blockbuster era based on patent monopolies, blinded the industry to the power of keeping customers satisfied. Today, it is facing paying a heavy price.
7. Can you elaborate on the role of regulatory authorities and the concerns you have about their independence and decision-making processes?
Regulatory Authorities are ‘Competent Authorities’, which are given delegated authority (powers) by a government for a defined area. In regulation of drugs, FDA is the competent authority in the US, and they are the sole arbiters in decision making. No other bodies, such as the CDC, WHO, or other self-appointed committees, can interfere. Country Regulatory Authorities have legally enforceable powers, through CFR Title 21 in the US, and DIRECTIVE 2001/83/EC OF THE EUROPEAN PARLIAMENT AND OF THE COUNCIL in the EU. Other countries will have similar legal powers.
What happened during COVID breached many of these laws. The International Coalition of Medicines Regulatory Authorities (ICMRA) infiltrated the formal country regulatory authorities beginning in 2012, at a meeting held by the WHO in Brazil. The current Chair, Ms Emer Cooke, is also Executive Director of the European Medicines Industry, a complete conflict of intertest.
This tells us that every regulatory authority in the world had their powers removed over the course of ICMRAs ‘networking’ activities, under the stewardship of successive Chair’s of the European Medicines Agency (EMA). How shocking is that?
8. What do you make of the claim that global organizations like the WHO and ICMRA have overstepped their mandates during the pandemic?
To say they overstepped their mandate doesn’t do justice to the scale of the steps they took. They grabbed power with no consideration for the harm it would cause. What is obvious, from the length of time it went on, that it was a careful constructed plan to maximise revenues earned and profits made from pharmaceutical products.
If I had to make a stab at the main perpetrator, it must be Bill Gates when he entered the industry in the late 1990s. One of my former colleagues joined the Gates Foundation in 2005 to work on the malaria vaccine. She didn’t stay long.
In 2011, Gates hired Trevor Mundel
“Trevor Mundel, President, Global Health
Trevor Mundel leads the foundation’s efforts to develop high-impact interventions against the leading causes of death and disability in developing countries. He manages the foundation’s disease-specific R&D investments in HIV, Tuberculosis, Malaria, Pneumonia, Enteric and Diarrheal Diseases, and Neglected Tropical Diseases. He also manages cross-cutting product development programs, including Discovery & Translational Sciences, Innovative Technology Solutions, Integrated Development, and Vaccine Development & Surveillance. This work relies on close collaboration with an international network of grantees and partners.
Prior to joining the foundation in 2011, Trevor was global head of development with Novartis and previously was involved in clinical research at Pfizer and Parke-Davis.”
Mundel did all the recruiting, including Ian Hudson, CEO, MHRA in 2019.
Then we have Anthony Fauci at the NIH and Jeremy Farrar at the Wellcome Foundation providing the research funding for their ‘pet’ projects. Next, there is GSKs Sir Richard Sykes, Chair of Uks vaccine task force working with MHRA in the UK to approve the injections, and Moncel Slaoui, 30 years at GSK, advising Donald Trump on Operation Warp Speed.
Then you had Lord Patrick Vallance, another GSK veteran executive team member, fanning the flames of fear on UK national TV, along with long term Gates collaborator, Sir Chris Whitty.
As if we haven’t heard enough of GSK, its former CEO, Sir Andrew Witty, has also been at it. This is taken from the WEF website and it begins:
“Sir Andrew Witty was named president, UnitedHealth Group, in November 2019, in addition to his role as chief executive officer of Optum. As UnitedHealth Group president, Sir Andrew is responsible for enterprise business strategy formulation, enterprise business development and partnerships, and oversight of enterprise research and development and clinical capacities.
Witty was named executive vice president, UnitedHealth Group, and chief executive officer, Optum, in March 2018. He is also a member of the Office of the Chief Executive and previously served as a UnitedHealth Group company director.
From 2008 to 2017, Witty was chief executive officer and a director of the leading pharmaceutical manufacturer GlaxoSmithKline (GSK). He joined GSK in 1985, and prior to being named CEO, served as president of GSK Europe.”
Is that enough overstepping their mandates for you? There’s a lot more, of course, as the dot joining continues.
9. How has public trust in the pharmaceutical industry and its regulatory bodies been affected by recent events, in your opinion?
As I pour over the various Substack’s sharing the horror stories of what has gone on, the impact on trust appears monumental. Certainly, the public will be looking toward alternative medicinal remedies outside of pharmaceutical solutions in a very big way. Although that is to be welcomed, a word of warning to the wise. Even natural remedies can be harmful if the supply chain that manufactures and distributes them is not of the required standard. This applies particularly in buying from internet sources.
10. You’ve highlighted the role of investment pressures in shaping pharmaceutical strategies. How has this dynamic influenced drug development and distribution?
The term ‘a dynamic’ is spot on. There has been a 40-year dynamic (downward spiral) operating, based on Big Pharma’s “Find It, File It, Flog It” strategy it invented in the 1980s to impress its investors.
Over four decades beginning when Big Pharma cast off its drug development assets, we saw the valley of death (no patented products getting to market) turn into the patent cliff (no new patented drug to become blockbusters). It got to the point where Big Pharma was in danger of having no patented products to sell, as they had given their old products to generic companies to copy the originals.
All they had/have left today is drugs for rare diseases, but at c. $400,000 for a single patient treatment, they were unaffordable and selling next to nothing.
The main rare disease drug is a gene modified cell therapy known as CAR T:
CAR T Cells: Engineering Patients’ Immune Cells to Treat Their Cancers
Novartis’ Kymriah is an example, approved by FDA August 2017. This is the package insert showing a black box FDA warning for Kymriah.
CRISPR gene editing technology is used to perform the genetic modification to cells.
This is the same technology used for the SARS-CoV-2 injections. Yes, it’s true, they were genetic engineering used on human beings
11. Many are concerned about the ethical implications of gene-editing technologies like CRISPR and base editing. What’s your perspective on their regulation and potential risks?
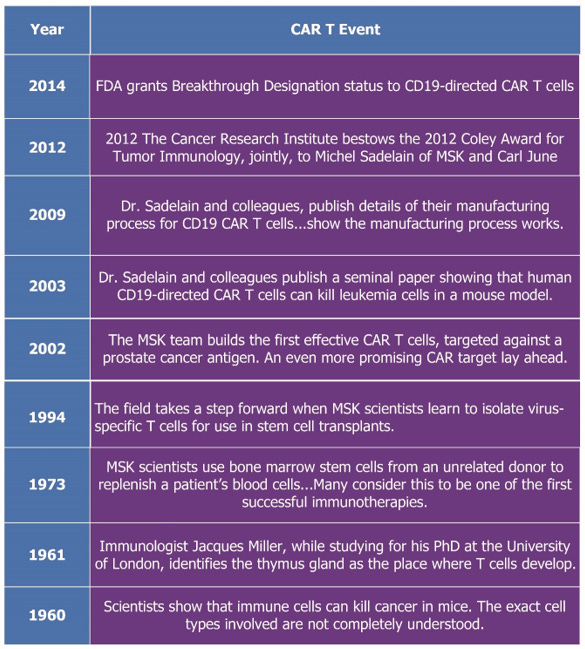
While researching my next book for Wiley, I happened across the history of CAR T (for rare blood cancers). This is what I found:
“Rare Diseases Targeted with CAR T Autologous Therapies
Since the early 1960s, research had been underway in a category of biologic products known as chimeric antigen receptor T (CAR T) therapy. With the renewed interest in biologics, attention turned to this previously unexplored territory. The history is summarized on the Memorial Sloan Kettering Cancer Center website titled CAR T Cells: Timeline of Progress.”
This is a timeline constructed from the history:
So, that’s a surprise. Nothing really happened to prove the manufacturing concept from 1960 to 2014, then FDA gave it breakthrough designation - It was on the market three years later!
This is the same therapy discussed in question 10. It was an experiment cooked up by research scientists with no experience of building or managing supply chains for even simple small molecule drugs, let alone these massively challenged ‘advanced’ therapies.
12. What lessons can be drawn from the history of penicillin’s development for modern drug development practices?
See: THE MOST IMPORTANT EVENT IN THE HISTORY OF MEDICINE
This is an excerpt to explain the ‘myth’ of penicillin:
In May 2017, a paper was published by Robert P. Gaynes, MD. Titled The Discovery of Penicillin – New Insights After More Than 75 Years of Clinical Use. It presents a dramatic re-interpretation of the penicillin story. It explains in detail how Governments, pharmaceutical companies, physicians, regulators, universities and other key stakeholders worked together collaboratively to bring penicillin to market.
This is in direct contradiction of the commonly held perception that penicillin, and its role in fighting infection for patients globally, was an accidental finding.
The paper explains that while Alexander Fleming discovered strong evidence that a mould he found in a culture dish on return from holiday was killing bacteria, he lacked the skillset to isolate the active ingredient within the mould.
It took over ten years before a team at Oxford University, headed up by Howard Florey, was able to isolate the active ingredient. They were able to make enough to produce small quantities for pre-clinical and clinical trials, which resulted in increased evidence that the compound could beat infection.
They lacked the skills, however, to make the kind of quantities that would be required to service the enormous market awaiting this breakthrough treatment. In June 1941, Florey and his fungal expert colleague Norman Heatley, travelled to the United States.
They meet with Charles Thom and Andrew Jackson Moyer, both with the US Department of Agriculture (forerunner to US FDA). Moyer suggested changes to the manufacturing process that resulted in “exponentially greater amounts of penicillin” being produced.
This led to successful mass production of penicillin to satisfy the demands of the World War II. The article comments
“Unprecedented United States/Great Britain cooperation for penicillin production was incredibly successful.” Moyer applied for a patent of the manufacturing process in 1945, which was granted in 1948.
The paper explains “The Fleming Myth” was down to an article in The Times following an interview with Fleming, but Florey and his staff refused to comment.
This myth has become hard coded into the industry psyche, perpetuating a public illusion that medicines are ‘discovered’ through serendipitous findings of a new molecular entity.
Our vitally important message here is that until a medicine can be manufactured to the scale necessary to supply the projected patient population, if does not exist.
Note also that the patent was awarded to Moyer in 1948 for the manufacturing process – there was no patenting of the molecular compound.
13. Do you see parallels between the handling of COVID-19 vaccines and other public health initiatives, such as the handling of antibiotics or antiviral drugs?
Yes, I do. The same Find It, File It, Flog It strategy of big pharma, and the focus on patent monopolies at the expense of patients, has resulted in antimicrobial resistance (AMR) remaining an unresolved threat to the world. The breakthrough of penicillin to fight infection was around 80 years ago. That’s a long time for microbes to find their way around the best antibiotic we have today. To top it all, the WHO has the effrontery to put this on its website:
“21 November 2023
Key facts
Antimicrobial resistance (AMR) is one of the top global public health and development threats. It is estimated that bacterial AMR was directly responsible for 1.27 million global deaths in 2019 and contributed to 4.95 million deaths
The misuse and overuse of antimicrobials in humans, animals and plants are the main drivers in the development of drug-resistant pathogens.” Read more here: Antimicrobial resistance
That is the same WHO that helped inflict the greatest medical experiment ever carried out on earth.
In terms of antivirals, the COVID scam taught us that Big Pharma was pushing its own ‘magic’ remedies at the expense of existing generic drugs with a long history of safety, and strong indications of efficacy, in humans. Ivermectin is a case in point.
The truth is that public health has become public disease, where the focus is on one size fits all epidemiology-based solutions to the spread of disease. Public heath should be about clean, chemical-free water, nutritious food, a healthy environment and all the other attributes that contribute to public wellbeing. As it is today, the focus is on preventing diseases that would not take hold if public health were properly executed. Finally, it was misuse of epidemiology data by Neil Ferguson at Imperial College, that drove much of the pandemic panic. That must never happen again.
14. You’ve been outspoken about conflicts of interest in the pharmaceutical industry. What reforms would you propose to address these issues?
Changes to FDA funding in recent years has created a major conflict of interest. It started in 1992, with the Prescription Drug User Fee Act, as amended. This meant that pharmaceutical companies were required to pay for their regulatory ‘services.’
“Latest News:
The FY 2025 PDUFA program fee invoices were emailed on Thursday, August 15, 2024. Full payment of the invoice is due on October 1, 2024. If you do not receive your invoice by August 19, 2024, please contact PDUFA User Fee staff at CDERCollections@fda.hhs.gov.
The FY 2025 PDUFA fee rates were published in the Federal Register (FR) on July 31, 2024. For more information regarding the FY 2025 fee rates, please see the FR notice.
On September 30, 2022, the President signed into law the FDA User Fee Reauthorization Act of 2022, which includes the reauthorization of the Prescription Drug User Fee Act (PDUFA VII) from fiscal year (FY) 2023 through 2027. Additional information can be found on the PDUFA VII web page.”
PDUFA was then followed by GDUFA:
Generic Drug User Fee, as amended.
Fees are now charged by the European Medicines Agency, see: https://www.dlrcgroup.com/ema-updated-fees-what-does-this-mean-for-my-applications/ which begins:
“Why Are the Fees Changing?
The overhaul of the fees legislation has been driven by the increase in workload at the EMA and the Agencies across the national Member States. Primarily driven by the COVID-19 pandemic and the subsequent initiatives and activities resulting from it, the fee increases are seen to be necessary to ensure the Agencies can continue to fulfil their obligations and commitments.
The regulatory environment is ever-changing. To meet the ever-increasing demands placed on them, sufficient funding needs to be available for the Agencies to maintain an appropriate level of highly skilled expertise and infrastructure, enabling them to deliver the high standards expected for medicinal products intended for the European market.
As a result, the fee structure has been significantly over-hauled. This establishes a single regulatory framework with a streamlined fee system that is flexible enough to be adjusted when required moving forward.”
So, the first reform to be recommended is to return the funding of regulatory authorities from the public purse. Regulators cannot regulate if its paymaster is the regulatee.
Second, is for governments to require regulatory authorities to place supply chain integrity front and centre of the drug licensing processes. This will require inspections to cover the pharmaceutical or biopharmaceutical supply chain from beginning (starting materials) to end (finished product in the hands of patient or clinician). The rationale is that errors and omissions can occur at any point in a supply chain. Therefore, it is the supply chain overall system that should be inspected, rather than stand alone facilities. This will need re-training of regulatory staff and other actions. However, the impact on patient safety would be nuclear, in driving the right behaviours in those developing drug supply chains. The eventual objective would be for companies to build integrity into the supply chain themselves, rather than be told what to do.
So, the second, and only other reform put forward at this stage, is to drive supply chain re-integration by regulators unearthing the deep issues that exist in today’s highly outsourced supply chains and pressing for remediation by product license holders at the ‘systems’ level.
15. What are you currently focused on, and how can readers stay in touch with your work to learn more about your insights and advocacy?
It’s one word – education.
In May 2019, I hosted this conference in Wales and wrote a white paper in summary:
Medicines for the 21st Century: Safe, better, cheaper
This is taken from the white paper:
Aim
This paper is presented in the context of rapidly increasing volume and intensity of calls for major reform of companies developing and supplying medicines into healthcare systems (pharmaceutical companies). The modus operandi of those companies appears to be working against the best interests of those using their products – patients and healthcare professionals.
On May 8th, 2019, a group of clinicians, patients, representatives of relevant charities, experts in product development, legal, regulatory and supply chain specialists gathered together in Wales, with the aim of examining that claim, based on facts and evidence.
The day was organised in conference format, titled “MEDICINES FOR THE 21st CENTURY: Safe, Better, Cheaper”. It involved in-depth dialogue and transfer of knowledge, over three panel sessions, between invited attendees and panel members, considering issues and opportunities in relation to safe medicines, better medicines and cheaper medicines. Proceedings over the day were recorded on video, and live polling was used to collect inputs from those in attendance.
I had asked Janet Woodcock MD, then Director, FDAs Center for Drug Evaluation and Review (CDER) to record the Keynote address, which she kindly agreed to do, here:
Janet Woodcock MD Keynote Address.
Dr Woodcock contributed a section on regulatory modernization for my previous Wiley book, some 15 years earlier. (In my opinion, she received unfair criticism for the opioid crisis in the US, when there was no way of stopping the pharmaceutical companies on the golden path to profit).
The inauguration speech was delivered by the former first minister for Wales, Carwyn Jones.
The paper continued on:
“Medicines making is broken – experts, patients and healthcare professionals to gather in Wales to make things better”
Backdrop
The above is taken from the press release announcing a conference which was held on 8th May 2019, involving a wide variety of stakeholders in the medicines (pharmaceutical) industry, including clinicians, patients, representatives of relevant charities, experts in product development, legal, regulatory and supply chain specialists.
Attendees were curated based upon their interest and ability to contribute. The event was held at the UK’s longest established science centre, Techniquest, located in Cardiff Bay. The aim was to explore our prima facie conclusion also reached by an increasing number of today’s industry commentators – the system by which medicines are developed and commercialised is not fit-for-purpose, alternatively termed ‘broken’.
The conference, titled MEDICINES FOR THE 21st CENTURY: Safe, Better,
Cheaper, focused on open dialogue and information exchange, following the programme below:
• Introduction to the day - broadcaster Clare Forestier.
• Inauguration speech - former First Minister of Wales, Carwyn Jones.
• Keynote Address (pre-recorded) - Dr Janet Woodcock, Director, Center for Drug Evaluation and Research (CDER), FDA.
• Overview of sessions and 20th century medicines paradigm - Hedley Rees, managing consultant, PharmaFlow
• Panel sessions:
o Safe medicines
o Better medicines
o Cheaper medicines
• Closing remarks and next steps
Input from attendees was collected using Sli.do (proprietary live-polling software), provided by local college (Bridgend) Head of Media, Dr Scott Morgan. We used #meds421C and #PatientsNotPatents to share comments on Twitter.
The next Wiley book covers this in more detail.
My next focus is to do it on a larger scale, here:
The current plan is to hold it during Q2 2025, following the publication of Wiley’s ‘Transforming the Pharmaceutical Supply Chain’, by Hedley Rees.
That’s what I’m up to at the moment.
Readers wishing to stay in touch with what I’m doing are best subscribing to INSIDE PHARMA. There are also the self-published books above, for those wanting to educate themselves on how the whole SARS-CoV-2 scam came to life, and help bring on its death.
I appreciate you being here.
If you've found the content interesting, useful and maybe even helpful, please consider supporting it through a small paid subscription. While everything here is free, your paid subscription is important as it helps in covering some of the operational costs and supports the continuation of this independent research and journalism work. It also helps keep it free for those that cannot afford to pay.
Please make full use of the Free Libraries.
Unbekoming Interview Library: Great interviews across a spectrum of important topics.
Unbekoming Book Summary Library: Concise summaries of important books.
Stories
I'm always in search of good stories, people with valuable expertise and helpful books. Please don't hesitate to get in touch at unbekoming@outlook.com
For COVID vaccine injury
Consider the FLCCC Post-Vaccine Treatment as a resource.
Baseline Human Health
Watch and share this profound 21-minute video to understand and appreciate what health looks like without vaccination.







Vaccines in general are depopulation. At their best they are slow kill. They have a long history of harming and killing. Gene therapy is the expected progression of a criminal industry. Frankenscience has revealed its ugly head, mad scientists playing God. The globalists themselves are on record speaking of their depopulation agenda. Bill Gates, Dennis Meadows, easily found on the internet. Speaking of their plans to lower the world’s population.
"If I had to make a stab at the main perpetrator, it must be Bill Gates when he entered the industry in the late 1990s. One of my former colleagues joined the Gates Foundation in 2005 to work on the malaria vaccine. She didn’t stay long.... In 2011, Gates hired Trevor Mundel"
Great interview, especially appreciate focus on long toxic history of Bill Gates as Rockefeller's heir to be overlord of food & drugs.